Key Points On Spin Quantum Number
- Quantum numbers give complete information about the electron in atom I, e., energy, position, size, shape and orientation of that orbital and the direction of spin. The direction of spin is described by the spin quantum number.
- The electron in an atom not only moves around the nucleus, but also spins about its own axis. This number gives information about the direction of spinning of the electron present in any orbital.
- The spin angular momentum is an intrinsic property, like rest mass and charge.
- The magnitude spin quantum number of an electron cannot be changed.
- The spin may lie in the 2s+1=2 orientation.
- Each type of subatomic particle has fixed spin quantum numbers like 0,1/2, 1, 3/2, etc.
- The spin value of an electron, proton, or neutron is 1/2.
- The particles having half-integral value of spin are called fermions.
- The particles having an integral value of spin are called bosons.
Types Of Quantum Number
The four-quantum number completely specifies an electron in an atom or gives a complete address of an electron in an atom. The four quantum numbers are:
- Principal quantum number \
- Azimuthal or secondary or angular quantum number \
- Magnetic quantum number \\)
- Spin quantum number \
What Is Pauli’s Exclusion Principle
Pauli’s exclusion principle is a rule that determines the restriction on the number of electrons that can occupy a specific atomic orbital. According to this principle: No two electrons in an atom can have the same values for the four quantum numbers.
For a given atomic orbital , the value of the principal, angular momentum, and magnetic quantum numbers are the same. So it can accommodate two electrons, provided the electrons have opposite spin.
Recommended Reading: Kuta Software Infinite Algebra 1 Arithmetic Sequences
N = Principal Quantum Number
The first quantum number, known as the principal quantum number, is given the symbol n. In order to describe a valid standing wave, n has to have integer values, but there’s an additional restriction on n as well. The value of n must be a positive integer value . In other words, n can never equal a negative integer. In fact, n can never even equal 0! The principal quantum number gives you two different clues as to what an electron wave looks like. First, it tells you how the electron density spreads out as you move away from the center of the atom. For electron waves with low principal numbers, like n = 1, the electron density is very thick right in close to the center of the atom, but then becomes rapidly thinner as you move out. In contrast, for electron waves with high principal quantum numbers, like n = 6, the electron density isn’t as thick near the center of the atom, but is spread quite a bit further out. In general, the higher the principal quantum number, the further away from the nucleus you’ll be able to detect a significant amount of electron density .
nFigure 6.13
Notice that as n increases, both the “size” of the electron wave and the number of nodes it has increase as well. As a result, the energy of an electron wave always increases with n.
What Is A Quantum Number
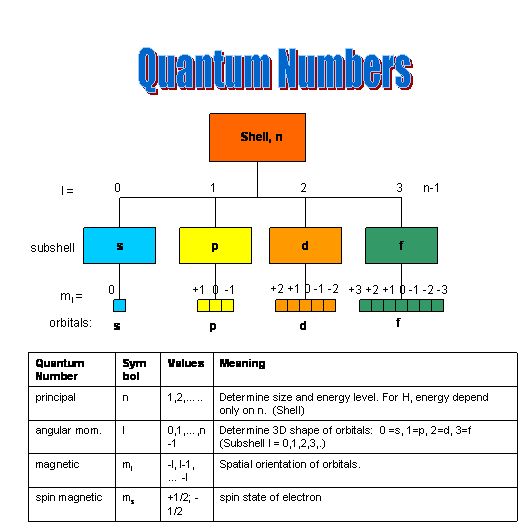
Quantum numbers are a set of values that we use to describe each electron in an atom. These numbers tell us about the electron’s energy, angular momentum, spin, and magnetic moment.
In a sense, the quantum numbers give us the electron’s address inside an atom.
There are four quantum numbers that we need to get a complete description of an electron within an atom:
- Principal quantum number
- Magnetic quantum number and
- Spin quantum number.
In the following sections, we will try to explain what each of these quantum numbers represents?
Also Check: What Do You Call Your Friends In Math Class
Background Of Quantum Numbers
The work of Broglie and Bohr have established how electrons have diverse discrete energy levels associated with their atomic radius. This model offered a comparatively, simpler spherical view. Moreover, this model by Bohr and Broglie indicated how the energy level of electrons is related to their principal quantum number. However, there are no numerical ways present in this model to classify additional behaviour of an electron in space.
Furthermore, Schrodingers equation offered three additional quantum numbers to describe an electrons behaviour in a more complicated multi-electron atom. This model was opposite to what Bohr and Broglie have done previously. Moreover, it opened new possibilities in the field of studying quantum numbers.
Additionally, based on these two models and further contributions from John Lennard-Jones and Slater, the Hund-Mulliken theory has been developed. Moreover, this theory is regarded as the most prominent system of nomenclature in the history of quantum mechanics.
Moreover, this nomenclature has incorporated Hund-Mullikens theory along with Bohrs energy levels, and observations made on electron spin on spectroscopy and Hunds rule.
The Spin Quantum Number
The spin quantum number describes the spin of a certain electron in an orbital. The spin is either +/- ½, which denotes either an up spin or a down spin. Each orbital can hold a max of two electrons, and if fully filled, the electrons cannot share the same spin direction. If electrons do not point in the opposite direction, then it would be a violation of the Pauli Exclusion Principle two electrons cannot share the same four quantum numbers. If the orbital is not fully occupied, then the electron can take on any spin direction. Convention usually assigns the up spin first in a non-fully occupied orbital.
Don’t Miss: What Is Physical Geography Ks3
The Orbital Angular Momentum Quantum Number
The orbital angular momentum quantum number \ determines the shape of an orbital, and therefore the angular distribution. The number of angular nodes is equal to the value of the angular momentum quantum number \. Each value of \ indicates a specific s, p, d, f subshell The value of \ is dependent on the principal quantum number \. Unlike \, the value of \ can be zero. It can also be a positive integer, but it cannot be larger than one less than the principal quantum number ):
Example \
If \, what are the possible values of \?
- Answer
-
Since \ can be zero or a positive integer less than ), it can have a value of 0, 1, 2, 3, 4, 5 or 6.
Example \
If \, how many angular nodes does the atom have?
- Answer
-
The number of angular nodes is equal to the value of l, so the number of nodes is also 4.
Valence And Core Electrons
- Electron present in the outermost orbit of the atom is called a valence electron and the electron present in the innermost orbit of an atom is called a core electron.
- The chemical properties of an element depend upon the valency of the element, and that is calculated from the number of valence electrons.
Exceptions
- Chromium : 1s22s22p63s23d54s1, Copper : 1s22s22p63s23p64s23d9
- These two are an exception because a full or half full d sub-level is more stable than a partially filled d sublevel, so an electron from the 4s orbital is excited and rises to a 3d orbital. In both cases, an electron moves from the 4s sublevel to produce a half-full 3d or completely filled 3d .
Know all about Solutions, its Components, Types, Properties here.
So, this is all about the Different Atomic Models. Get some practice of the same on our free Testbook App. Download Now!
Read Also: How Did Geography Make The Invasion Of Omaha Beach Difficult
Angular Momentum Quantum Number \\
The angular momentum quantum number, signified by \, describes the general shape or region an electron occupiesâits orbital shape. The value of \ depends on the value of the principal quantum number, \. The angular momentum quantum number can have positive values of zero to \\). If \, \ could be either \ or \.
High School Chemistry/quantum Numbers
|
|
We’ve spent a lot of this chapter talking about waves, and electron waves in particular. While most of us know what normal water waves look like, very few people have an understanding of what electron waves look like. In the last lesson, we talked about electron density, and how an electron wave could be thought of as representing the thickness or thinness of the electron density “fog” at any point in space within the atom. We considered the probability pattern for the electron in a hydrogen atom. Now let’s consider some more complicated atoms.
- Explain the meaning of the principal quantum number, n.
- Explain the meaning of the azimuthal quantum number, .
- Explain the meaning of the magnetic quantum number, ml.
Don’t Miss: What Does How Much Mean In Math Add Or Subtract
Azimuthal Quantum Number Or Angular Momentum Or Subsidiary Quantum Number
From the line spectrum, it was well observed that the spectrum has main lines and some fine lines. To explain the fine lines obtained in the spectrum, and was suggested that electrons in any shell of multiple electron atoms do not have the same energy as they move on a different path and have different angular momentum. Hence within the same shell, subshells or sub-energy levels are present.The azimuthal quantum number gives us various other information as well, like
The azimuthal number is denoted by the letter \, and the value of the subshell is an integral value that can range from \ to \.
For example, for the first shell \, \, \ can have only one value, i.e., \ and for the second shell \, \, \ can have two value, i.e., \ and \.
Depending upon the value of \, i.e., \, and \, the different subshells are designated as \, and \, respectively. These notations are the initial letters of the words, sharp, principal, diffused, and fundamental, formerly used to describe different spectral lines.
The number of subshells present in any principal shell is equal to the number of the principal shell or the principal quantum number. The possible subshell in the first four shells and their designation is summed up in the following table.
| Principal shell | |
| \ | \ |
The Pauli Exclusion Principle
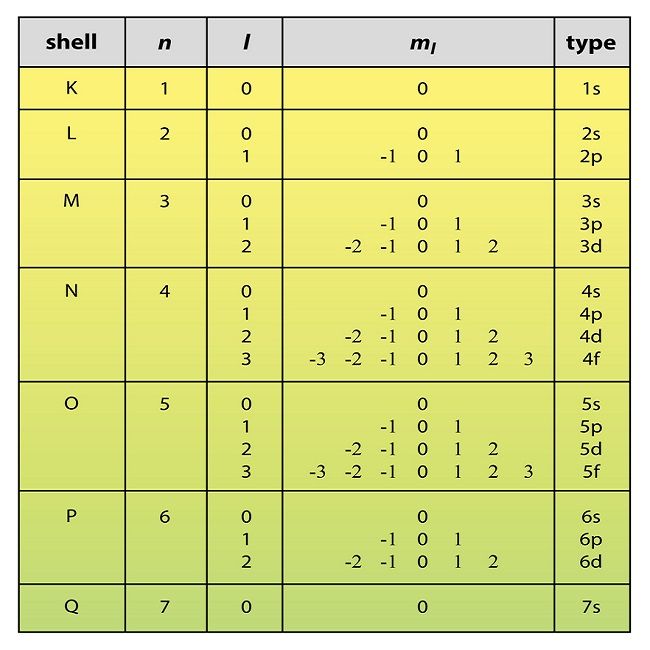
An electron in an atom is completely described by four quantum numbers: n, l, ml, and ms. The first three quantum numbers define the orbital and the fourth quantum number describes the intrinsic electron property called spin. An Austrian physicist Wolfgang Pauli formulated a general principle that gives the last piece of information that we need to understand the general behavior of electrons in atoms. The Pauli exclusion principle can be formulated as follows: No two electrons in the same atom can have exactly the same set of all the four quantum numbers. What this means is that two electrons can share the same orbital only if their spin quantum numbers ms have different values. Since the spin quantum number can only have two values (
Don’t Miss: Where’s My Water Cool Math Games
Spin Quantum Number \\
The spin quantum number describes the spin for a given electron. An electron can have one of two associated spins, \\) spin, or \\) spin. An electron cannot have zero spin. We also represent spin with arrows \ or \. A single orbital can hold a maximum of two electrons, and each must have opposite spin.
Limitations Of Quantum Numbers
There are some restrictions and limitations to quantum numbers. The accuracy with which they can predict the position and energy of electrons is governed by the following principles:
1. Paulis Exclusion Principle: This is a theory of quantum mechanics that was propounded by Wolfgang Pauli in 1925. According to this principle:
No two identical fermions can be in the same quantum state at the same time. For electrons in the same atom, this law states that no two electrons can have the same four quantum numbers. According to this principle, no two particles can stay in the same place at the same time. The particles which follow this principle are called fermions, such as electrons, atoms, neutrons etc. and the particles which do not obey this principle are called bosons, such as photons, gluons, gauge bosons.
2. Hunds Rule: According to this rule First, one electron is filled in all the sub-orbitals of any orbital and after that pairing starts, that is, pairing is made later, first one electron is filled in all the sub-orbitals. When an orbital is half full or completely full, then this orbital is comparatively more stable. Therefore, according to Hunds law, all the sub-orbitals of any orbital are filled with one electron first and their pairing starts.
This Hunds law is also called the law of maximum modalities.
Recommended Reading: What Is Naoh In Chemistry
The Electron Spin Quantum Number
Unlike \, \, and \, the electron spin quantum number \ does not depend on another quantum number. It designates the direction of the electron spin and may have a spin of +1/2, represented byâ, or â1/2, represented by â. This means that when \ is positive the electron has an upward spin, which can be referred to as “spin up.” When it is negative, the electron has a downward spin, so it is “spin down.” The significance of the electron spin quantum number is its determination of an atom’s ability to generate a magnetic field or not. /Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electron_Spin” rel=”nofollow”> Electron Spin.)
Example \
List the possible combinations of all four quantum numbers when \, \, and \.
- Answer
-
The fourth quantum number is independent of the first three, allowing the first three quantum numbers of two electrons to be the same. Since the spin can be +1/2 or =1/2, there are two combinations:
Example \
-
No, if the value of \ is positive, the electron is “spin up.”
Introduction To Quantum Numbers& Orbital Shapes
Quantum numbers are used to describe atomic orbitals, regions of space in which an electron can be found. From these numbers, we can determine the different properties of electrons in an atomic orbital. It is important to note that each electron will be unique to another, according to the Pauli Exclusion Principle. For this to be true, no two electrons in the same atom can have the same four quantum numbers.
Read Also: How To Work Out Standard Deviation Biology
Angular Momentum Quantum Numbers
- In spectroscopy: Angular momentum quantum numbers
The number l, called the orbital quantum number, must be less than the principal quantum number n, which corresponds to a shell of electrons. Thus, l divides each shell into n subshells consisting of all electrons of the same principal and orbital quantum numbers.
- In chemical bonding: Quantum numbers
number needed to specify an orbital is denoted l and called the orbital angular momentum quantum number. This quantum number has no role in determining the energy in a hydrogen atom. It represents the magnitude of the orbital angular momentum of the electron around the nucleus. In classical terms, as
Orbital Shapes The Angular Momentum Quantum Number
There are four different kinds of orbitals, which are named s, p, d and f orbitals. They each have a different orbital shape. An s-orbital is spherical with the nucleus at its center. A p-orbital is dumbbell-shaped and four out of five d-orbitals are cloverleaf shaped. The last d-orbital is an elongated dumbbell with a donut around its center.
The angular momentum quantum number describes the subshell, or the shape, of an orbital, and its allowable range is . There are four distinct shapes to remember: the s, p, d, and f orbitals. The value of l assigned to each subshell is based on the number of angular nodes . For s orbitals, which are spheres, there is no angular node, so l = 0. For p orbitals, which has electron density separated by one angular node, l = 1. Following this trend, d orbitals would have l = 2 and f orbitals would have l = 3, as they have two and three angular nodes, respectively.
lll
You May Like: What Is Duplication In Biology
The Magnetic Quantum Number
The magnetic quantum number \ determines the number of orbitals and their orientation within a subshell. Consequently, its value depends on the orbital angular momentum quantum number \. Given a certain \, \ is an interval ranging from \ to \, so it can be zero, a negative integer, or a positive integer.
Example \
Example: If \, and \, then what are the possible values of \?
- Answer
-
Since \ must range from \ to \, then \ can be: -2, -1, 0, 1, or 2.
Principal Quantum Number \\
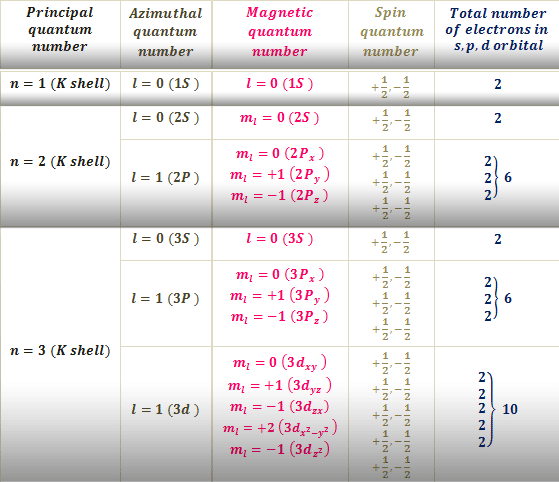
The principal quantum number, signified by \, is the main energy level occupied by the electron. Energy levels are fixed distances from the nucleus of a given atom. They are described in whole number increments . At location \, an electron would be closest to the nucleus, while at \ the electron would be farther, and at \ farther yet. As we will see, the principal quantum number corresponds to the row number for an atom on the periodic table.
Recommended Reading: What Is Theorem In Math
Get Quantum Numbers Worksheet Answer Key Worksheet
Quantum numbers chemistry worksheet. Quantum numbers this is our final way to describe the location of an electron. N = 5, = 0. First primary quantum number n size of electron cloud n 1 up to in reality n 1 7second azimuthal or angular momentum quantum number l shape of electron cloud l 0 up to n 1.
Quantum numbers worksheet name _____ 1. Also download free pdf chemistry class 11 assignments and practice them daily to get better marks in tests and exams for grade 11. Element 1s 2s 2p 3s 3p 4s 3d quantum numbers 1.
Free Download
*Click “Save Image” to View FULL IMAGE
Name the orbitals described by the following quantum numbers a. N = 3, l = 1 c. N = 3, l = 0 b.
N 3 l 0 b. In this worksheet, we will practice using quantum numbers to describe an electron within an atom. F g h a b d f g the quantum.
Give the n and l values for the following orbitals. N = 3, l = 0 b. N = 5, = 0.
What is the correct order from lowest to highest energy of these electrons? Specifies the orientation of the spin axis of an electron. Quantum numbers worksheet name _____ 1.
N = 5, l = 0 3. The principal quantum number, n, indicates the _energy level_. Quantum numbers worksheet key 1.
N = 3, l = 2 d. Todays worksheet will assist your trainees get a head start on learning more about verbs as well as their numerous tenses and kinds. State the four quantum numbers, then explain the possible values they may have and what they actually represent.
Free Download