Comparing To A Reference
It is typical to record spectrum of both the sample and a “reference”. This step controls for a number of variables, e.g. infrared detector, which may affect the spectrum. The reference measurement makes it possible to eliminate the instrument influence.
The appropriate “reference” depends on the measurement and its goal. The simplest reference measurement is to simply remove the sample . However, sometimes a different reference is more useful. For example, if the sample is a dilute solute dissolved in water in a beaker, then a good reference measurement might be to measure pure water in the same beaker. Then the reference measurement would cancel out not only all the instrumental properties , but also the light-absorbing and light-reflecting properties of the water and beaker, and the final result would just show the properties of the solute .
A common way to compare to a reference is sequentially: first measure the reference, then replace the reference by the sample and measure the sample. This technique is not perfectly reliable if the infrared lamp is a bit brighter during the reference measurement, then a bit dimmer during the sample measurement, the measurement will be distorted. More elaborate methods, such as a “two-beam” setup , can correct for these types of effects to give very accurate results. The Standard addition method can be used to statistically cancel these errors.
Microplastics A Big Problem For The Environment
Researchers use Raman spectroscopy to characterize microscopic pieces of plastic that invade our environment. These materials, those both engineered and those that are the product of decomposition, might pose health hazards. Scientists use Raman spectroscopy to trace the trail of microplastics that are becoming a greater threat to our surroundings.
Applications Of Uv Vis Spectroscopy
In research, ultraviolet / visible spectroscopy is used more commonly than in detection. Through first reacting the sample to bring the metal into solution as an ion, the trace metal content of an alloy, such as manganese in steel, can be determined.
A common technique for quantitative analysis of analytes in QA / QC, analytical research and government regulatory laboratories is UV-Visible spectrophotometry. The fundamentals of the approach are learned in school, such as Beers Law. UV-Visible Mid-range to Upper-end Spectrophotometers are typically used in research laboratories, including university and industrial laboratories.
The ion is then complexed or made to react so that it can be measured in a shape, such as manganese as the manganate ion. When the spectrum is registered, the absorbance is the most valuable bit of information since the concentration of the solution can be determined if the absorption coefficient of the chromophore is known, and thus the mass of the metal in the sample.
Don’t Miss: Who Are Paris Jackson’s Biological Parents
Fiber Optic Cables And Spectrometry
In absorbance spectrometry, a sample is placed in a cuvette, which is inserted into the spectrometer. NIR and IR spectrometry often analyze sources that cannot be placed in a cuvette, so a fiber optic cable is used instead. Fiber optic cables, made of glass or plastic, transport light from an external source to the photodiode of a spectrometer using total internal reflection. Glass fiber optic cables are attenuated by absorption and scattering factors. Water bands due to minute amounts of water vapor in the glass cause absorption, and scattering occurs when light bounces off molecules within the glass. To reduce light absorption, the refractive index of the fiber optic core must be greater than the refractive index of the cladding. Fiber optic cables are most often applied to NIR and IR studies, which frequently have sources that cannot be transferred to a cuvette. The most common fiber optic wavelengths are 850 nm, 1300 nm, and 1550 nm.
Uv/vis Spectroscopy And Image Analysis For The Quantification And Classification Of Biofuels
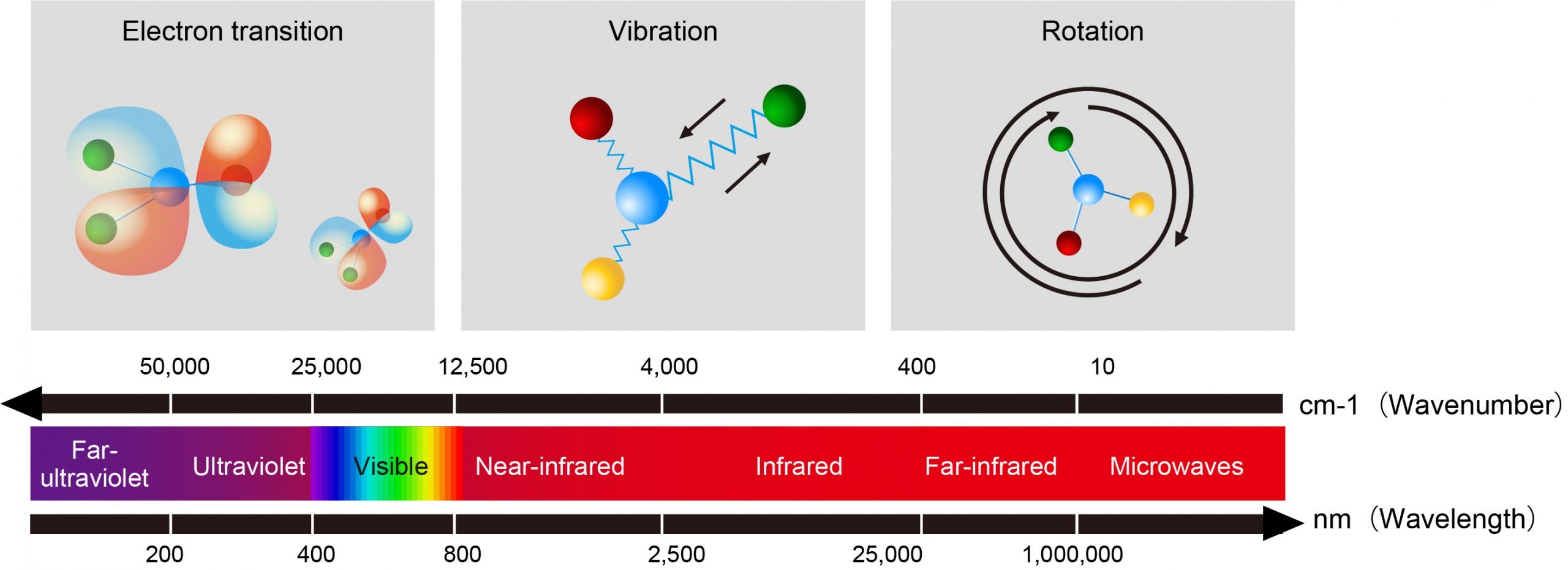
The absorption of radiation in the ultraviolet/visible region results from the excitation of bounding electrons. The UV/Vis radiation has enough energy to promote electronic transitions, and this is the main principle investigated by UV/Vis absorption spectroscopy. This technique is very useful in identifying functional groups in a molecule because a correlation between the absorption bands and the functional group can be done. While inorganic compounds normally absorb light in the visible part of the spectrum, organic molecules usually present some functional groups capable of absorbing radiation from UV light sources. These functional groups must be unsaturated or have a heteroatom with non-bonding electrons, such as oxygen, sulfur, or halogens. Table 3 shows some functional groups and the radiation wavelength absorbed .
According to the Lambert-Beer law, the intensity of the absorption of radiation by the species present in the sample is directly proportional to its concentration in the system. Thus, quantitative determination of compounds containing absorbing groups can be easily made. UV/Vis spectroscopy is widely used for many applications, including in the biofuel area, since it is low cost and allows the analyst to perform qualitative and quantitative analysis in a fast and reliable way.
Recommended Reading: Algebra 1 Eoc Answers 2015
What Is Uv Vis Spectroscopy And How Does It Work
UV-Vis is a quick , convenient, and inexpensive way of determining the solution concentration of an analyte. In UV-Vis, a beam travels through a solution in a cuvette with a wavelength ranging between 180 and 1100 nm. The sample absorbs this UV or visible radiation in the cuvette.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Nuclear Magnetic Resonance And Biofuel Applications
The theory of nuclear magnetic resonance spectroscopy was proposed by Pauli in 1924. He suggested that when certain atomic nuclei have spin and a nuclear magnetic moment, in the presence of an applied magnetic field , they could be induced to absorb energy and change their spin orientation with respect to this applied field .
The independent works of Felix Block and Eduard Purcell in 1946 experimentally showed that by absorbing electromagnetic radiation in the presence of an intense magnetic field, the nucleus experiences an unfolding of its spin state energy levels, as shown in Figure 8, which is dependent on the spin itself. In addition, the energy absorbed must equal the energy difference between the two states involved in this case, the energy absorption is a quantized process . The stronger the applied magnetic field, the greater the energy difference between the two inverse spin states, as shown in Figure 9.
Figure 8.
= 3/2 in the absence and in the presence of an applied magnetic field .
Figure 9.
Spin state energy separation as a function of the increase in the magnetic field.
Figure 10.
Predicted 1H NMR spectra of the ethanol molecule.
Figure 11.
Time domain. FID Curve for 1H NMR for acetone spectra created using the Fourier transformation to convert the time domain to a frequency domain.
Figure 12.
Read Also: Segment And Angle Addition Postulate Worksheet Answers
Relative Atomic Mass Lab Report
Explain how the molarity of the standard solution was calculated in the experiment – 0.1M of NaOH is required, this equation will be used: Concentration = moles volume This will be rearranged to find the moles needed to carry out the experiment. The concentration of the experiment using NaOH is 0.1M so we just need to rearrange the equation to find the molarity. 0.1x 0.250 = 0.0250 moles Number of moles = mass RFM 0.0250 = mass 40 0.0250 x 40 + 1g Explain how this enabled you to accurately calculate the molarity of each acid used in the titrations – Molarity of the acid = molarity of the alkali x volume of the alkali volume of acid Firstly we will need to add up all of the volumes found within the titration to find an average:13.10+13.20+13.10= 13.13 Molarity of Ethanoic acid = 0.1 x 25.00 = 0.190 mol dm-3 13.13 Molarity of Hydrochloric acid = 1.0 x 25.00 = 0.077 mol dm-3 32.53
Need A New Nmr Spectrometer For Your Lab
If you’re looking for an NMR spectrometer for your school or lab, Nanalysis is a great option. Nanalysis’ NMReady provides an easy solution for accessibility and maintenance with its compact and portable design.
For more information about NMR applications or benchtop NMR spectrometers for your classroom, contact Nanalysis today.
Also Check: How To Find Ksp Chemistry
Porosity Of Organic Coatings
The EIS technique can be used to evaluate the porosity of organic coatings. If the coating is of good quality, no significant changes occur on the specimen surface before measuring the anodic polarisation curves. In contrast, very porous films will already have failed after measurements of the corrosion potential .
Determining porosity is difficult because of the small size of the defects. By using electrochemical measurements, porosity can be estimated from the electrochemical values. Assuming that the coating is electrochemically inert at low anodic overpotentials, Matthes et al. established an empirical equation to estimate the porosity of the coating :
R. Ilangovan, … S. Renganathan, in, 2021
A Potted History Of Spectroscopy
- Sir Isaac Newton
It was 1666 when Newton showed that the white light from the sun could be dispersed into a continuous series of colours. Newton introduced the word “spectrum” to describe this phenomenon and it was this analysis of light that was the beginning of the science of spectroscopy.
In 1800, William Herschel demonstrated that the sun’s radiation extended into the infrared, and in 1801 Johann Wilhelm Ritter made similar observations in the ultraviolet.
However, it was the achievements of Joseph Fraunhofer in the early 1800’s that provided the quantitative basis for spectroscopy. Fraunhofer extended Newton’s discovery by observing that the sun’s spectrum, when sufficiently dispersed, was crossed by a large number of fine dark lines, now known as Fraunhofer lines.
Fraunhofer also developed the diffraction grating, an array of slits, which disperses light in much the same way as a glass prism, but with the advantage of being able to directly measure the wavelength of the diffracted beam. Earlier, Thomas Young had demonstrated that a light beam passing through a slit emerges in a pattern of bright and dark fringes. Fraunhofer extended these studies to the case of two, three and many closely spaced slits, and the transmission grating was created. With this, he was able to directly measure the wavelengths of spectral lines.
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
\ Basic Components Of Spectroscopic Instruments
The spectroscopic techniques in Table \ and Table \ use instruments that share several common basic components, including a source of energy, a means for isolating a narrow range of wavelengths, a detector for measuring the signal, and a signal processor that displays the signal in a form convenient for the analyst. In this section we introduce these basic components. Specific instrument designs are considered in later sections.
Note
You will find a more detailed treatment of these components in the additional resources for this chapter.
Application Of Emission Spectroscopy:
- A laboratory-based hard x-ray monochromator is used for high-resolution applications exploiting the X-ray emission spectroscopy.
- A standard application is also near edge structure measurements as the atoms decay to the ground stage, exploiting X-ray absorption. The emitted radiation usually passes through the monochromator used to isolate the specific characteristic wavelength for this specific analysis.
- Emission spectroscopy in AES or Atomic-emission spectroscopy generally exploits the quantifiable optical emission measurement starting as of excited atoms to assess concentration and its emission spectra. Extra specifics concerning the electronic and geometric structure of transition metals could also be investigated and analyzed.
- Spectroscopic measures based on emission spectrum and nonlinear x-ray spectroscopy are used to analyze a different type of transition such as metal compounds in inorganic chemistry, characterization of catalysis, and materials science application.
Recommended Reading: Lesson 9.5 Practice B Geometry Answers
Essay On Column Chromatography
DetectorThis detector is used for measurement of specific physical and chemical properties of the column effluent. The most common detector used in pharmaceutical analysis is UV, which allows monitoring and continuous measurement of the UV absorbance at a selected wavelength. Appearance of the analyte in the detector flow-cell causes the change of the absorbance. If the analyte absorbs greater than the background a positive signal is
What You Need To Know About Carbon Nanodots
Carbon nanodots are tiny particles made of carbon on the nanometer scale. Scientists can make it from various sources, such as bulk carbon or carbohydrates. They can even make it from biomass, which is a total mass of organisms. The cost of preparation can be cheap since these particles are easy to synthesize.
Scientists produce carbon nanodots as stacks of a few graphene layers in a continuous two-dimensional carbon honeycomb. Due to the confined size, carbon nanodots have finite band-gap that can absorb and emit light.
Carbon nanodots are important because of its photoluminescence properties. Scientists can tune the color of the fluorescence from carbon nanodots by modifying its size and surface chemistry. Researchers use spectrofluorometers to measure the photoluminescence of these materials.
Medical practitioners introduce these nanosized materials into biological cells to color the cells and track the biological components. Manufacturers also use carbon nanodots in display technology.
Don’t Miss: Define Movement In Geography
The Industrial Liaison Team At Diamond
Would you like to know more about X-ray spectroscopy and how you can apply it to your research? Do you perhaps have a structural problem that you are unable to solve in your lab or a material you wish to find out more about? Then please get in touch with the Industrial Liaison Team at Diamond.
The Industrial Liaison team at Diamond is a group of professional, experienced scientists with a diverse range of expertise, dedicated to helping scientists and researchers from industry access the facilities at Diamond.
Were all specialists in different techniques and have a diverse range of backgrounds so were able to provide a multi-disciplinary approach to solving your research problems.
We offer services ranging from full service a bespoke experimental design, data collection, data analysis and reporting service through to providing facilities for you to conduct your own experiments. Were always happy to discuss any enquiries or talk about ways in which access to Diamonds facilities may be beneficial to your business so please do complete an enquiry form or give us a call on 01235 778797. You can keep in touch with the latest development by following us on Twitter or
How Spectroscopy Is Used
Far from being a specialised, unique field, spectroscopy is integral to a variety of disciplines. While it provided a theoretical backing to early quantum research in radiation and atomic structure, it also has a staggering number of other applied uses magnetic resonance imaging and X-ray machines utilise a form of radio-frequency spectroscopy, we measure the unique makeup and physical properties of distant astral bodies through their spectra and wavelength, and its even used to test doping in sports.
The different types of spectroscopy are distinguished by the type of radiative energy involved in the interaction. In many applications, the spectrum is determined by measuring changes in the intensity or frequency of this radiative energy. The types of spectroscopy can also be distinguished by the nature of the interaction between the energy and the material. Examples include:
Astronomical spectroscopy
This type of spectroscopy is chiefly concerned with the analysis of objects in space. From simple spectroscopic analysis of an astronomical object, we can measure the spectrum of electromagnetic radiation and determine its wavelength. This can tell us about the objects chemical composition , temperature, distance and speed .
Absorption spectroscopy
Biomedical spectroscopy
Energy-dispersive X-ray spectroscopy
Read Also: Write The Segment Addition Postulate For The Points Described
Spectroscopy Used To Find Life In Hidden Environments
Like water treatment plants, researchers use fluorescence spectroscopy to measure dissolved organics in glacial ice. This helps to determine if life exists or existed at one time below the polar ice caps.
Scientists used a HORIBA Scientific Aqualog spectrofluorometer in a number of such experiments in Antarctica. They used the Aqualog to search for the fingerprints of microorganisms.
Knowledge like this also adds to our understanding of the possibilities of life on other, frozen planets.
Basic Principles Of Local Deep
The local-DLTS technique is based on the contact of a sharp conductive tip with the outer surface of an insulator layer, such as SiO2, on a semiconductor such as SiC. In this scenario, a miniature MOS capacitor is produced between the sample and the tip, the capacitance of which, Cs, can be varied by applying a voltage, V, to the sample. This voltage alternates between Va and Vb at a frequency, fp, such that the time span over which V equals Vb is tpw. As a result, a pulsed rectangular waveform is generated, as shown in Fig. 11.3A. The change in capacitance induced by varying the voltage,
, where EB is the binding energy of the electron, h is the known energy of the incident photon, and Ekf is the measured kinetic energy of the emitted electron. Two key photoemission spectroscopy methods that have provided key insights into topological insulator properties are x-ray photoemission spectroscopy , which elucidates the elemental and chemical state composition of the material surface, and angle-resolved photoemission spectroscopy , which measures the density of single particle excitations in the reciprocal space of a solid, allowing for simultaneous measurement of both energy and momentum of electrons in the solid. By directly relating the kinetic energy of emitted electrons to both EB and the crystal momentum k of the solid to resolve occupied states in energy-momentum space, ARPES provides a unique capability of imaging the electronic band structures of materials .
Recommended Reading: Fsa Algebra 1 Eoc Practice Test Answers
Wave Properties Of Electromagnetic Radiation
Electromagnetic radiation consists of oscillating electric and magnetic fields that propagate through space along a linear path and with a constant velocity. In a vacuum electromagnetic radiation travels at the speed of light, c, which is 2.997 92 × 108 m/s. When electromagnetic radiation moves through a medium other than a vacuum its velocity, v, is less than the speed of light in a vacuum. The difference between v and c is sufficiently small that the speed of light to three significant figures, 3.00 × 108 m/s, is accurate enough for most purposes.
The oscillations in the electric and magnetic fields are perpendicular to each other, and to the direction of the waves propagation. Figure \ shows an example of plane-polarized electromagnetic radiation, consisting of a single oscillating electric field and a single oscillating magnetic field.
Figure \: Plane-polarized electromagnetic radiation showing the oscillating electric field in red and the oscillating magnetic field in blue. The radiations amplitude, A, and its wavelength, , are shown. Normally, electromagnetic radiation is unpolarized, with oscillating electric and magnetic fields present in all possible planes perpendicular to the direction of propagation.
where Am is the magnetic fields maximum amplitude.
Note
This change in wavelength as light passes between two media explains the refraction of electromagnetic radiation shown in Figure \.
Example \
Solution
Exercise 10.1