Is Water Is A True Solution
Answer: True Solution is a homogeneous mixture of two or more materials with a particle size of less than 10-9 m or 1 nm dissolved in the solvent. Example: Simple sugar solution in water. Particles can not be isolated from true solutions by using filter paper which is also not apparent to the naked eye.
Erin Brokovich And Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr used by Pacific Gas & Electric to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brokovich , Erin and lawyer Edward Masry sued PG& E for contaminating the water near Hinckley in 1993. The settlement they won in 1996$333 millionwas the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Figure 1. Erin Brockovich found that Cr, used by PG& E, had contaminated the Hinckley, California, water supply. The Cr ion is often present in water as the polyatomic ions chromate, CrO42 , and dichromate, Cr2O72 .
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr or Cr forms. Cr, an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Dig Deep On Practice Problems
Just doing lots of practice problems will not necessarily make you a better problem solver. You will never see an exam problem that looks exactly like a practice problem, so doing every problem possible is not a good strategy. Instead, when you work out a practice problem we have given you, make sure that you can explain why and when you would make each step in your solution. Be able to explain
- why certain information is useful to you
- why a piece of information might be unnecessary
- what conversions you need to make so that you can use information correctly
- why you are using a specific formula
- how you can rearrange a formula to find a new parameter
- why you need to consider a particular reaction
- when would you be able to make any assumptions you are making
- what structures are useful to understand
It is easy to fall into the trap of reading through a solution key and thinking it makes sense. But unless you can justify each step with more than a just because statement, it will be difficult to apply those skills to another problem.
You May Like: What Is The Molecular Geometry Of Ccl4
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Compounds Containing A Metal Ion With A Variable Charge

Most of the transition metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ , and the two corresponding compound formulas are FeCl2 and FeCl3. The simplest name, iron chloride, will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron chloride and iron chloride, respectively. Other examples are provided in Table 4.
| Table 4. Names of Some Transition Metal Ionic Compounds | |
|---|---|
| Transition Metal Ionic Compound | |
| SnF4 | tin flouride |
Also Check: Theory Of Everything Geometry Dash Song
Study Chemistry When You Are Awake
We all tend to put off things that are difficult, but this means that you might end up studying chemistry at the very end of the day when you are already worn out and too tired to think well. And, if you never practice then it will never get easier!
Instead, try setting aside some time each day when you know you will be alert and ready to go. It doesnt have to be a huge block of time, but that way you will at least get in some quality time to bond with your chemistry.
Theoretical Actual And Percent Yields
The percent yield is a comparison between the actual yieldwhich is the weight of the intended product of a chemical reaction in a laboratory settingand the theoretical yieldthe measurement of pure intended isolated product, based on the chemical equation of a flawless chemical reaction, and is defined as,
When more than one reactant participates in a reaction, the yield is usually calculated based on the amount of the limiting reactant, whose amount is less than stoichiometrically equivalent to the amounts of all other reactants present. Other reagents present in amounts greater than required to react with all the limiting reagent present are considered excess. As a result, the yield should not be automatically taken as a measure for reaction efficiency.
In their 1992 publication General Chemistry, Whitten, Gailey, and Davis described the theoretical yield as the amount predicted by a stoichiometric calculation based on the number of moles of all reactants present. This calculation assumes that only one reaction occurs and that the limiting reactant reacts completely.
Read Also: Eoc Fsa Warm Ups Algebra 1 Answers
Pressure And Free Energy
Organic Chemistry Is Three Dimensional
You will find that nearly all of the study skills developed in general chemistry are just as applicable in organic: you still have to put in the time for concepts to marinate, you have to dig deep in problems, and you have to be on constant vigilance to ask why. However, in organic chemistry, there is a new visual component to take into account: it is essential to begin viewing molecules three dimensionally , since the 3D structure greatly impacts the actual chemistry. To start visualizing these structures use a model kit to build molecules every time you do organic chemistry . Bring the model kit to section. Your models will reveal important properties of the molecules, like the spatial relationships between different atoms, or how easily a bond can rotate. Keep the model kit on you at all times and use it!
Above all, keeping trying!! Everyone learns at different speeds and in different ways. There are lots of resources here for you because we know you can do it with the right tools. If you dont know where to start just ask meet with one of the course TAs, tutors or professors. We are all here to help YOU SUCCEED!
Also Check: What Does Mole Mean In Chemistry
Common Names V Systematic Names
Many chemicals are so much a part of daily life that people know them by their familiar names. Ordinary cane sugar, for example, is more formally known as sucrose, but asking for it at the dinner table by that name will likely be a conversation stopper. Now imagine using its systematic name in the same context: Please pass the -D-glucopyranosyl—D-fructofuranoside! But saying sucrose would be quite appropriate if you needed to distinguish this particular sugar from the hundreds of other named sugars. And the only place you would come across a systematic name such as the rather unwieldy one mentioned above would be in scientific documentation in reference to a sugar that has no simple common name.
Many common chemical names have very old and intriguing origins, as the following two examples illustrate.
Most people associate the name ammonia with a gas with a pungent odor. While its systematic name, nitrogen trihydride , tells you its formula, what it will not tell you is the interesting history of its discovery. Smoke from burning camel dung condenses on cool surfaces to form a crystalline deposit, which the ancient Romans first noticed on the walls and ceiling of the temple that the Egyptians had built to the sun god Amun in Thebes. They named the material sal ammoniac, meaning salt of Amun. In 1774 Joseph Priestley found that heating sal ammoniac produced a gas with a pungent odor, which T. Bergman named ammonia eight years later.
The Benefits Of A Digital Inventory System
How exactly can a digital chemical inventory management system transform your research and save you money? By tracking your chemicals inventory, location, quantity, safety information, users, and regulatory compliance requirements, you have this data available at the touch of a button.
With a best-practices system in place, realtime data about your inventory becomes available to every relevant individual. You can look up exactly what you have, where its located, how much you use and how often, when to reorder, and how to use, store, and dispose of the chemical safely.
In daily use, a best-practices chemical inventory system can shave hours off inventory searches and dollars off redundant orders. In emergencies, a remotely accessible, real-time inventory list can serve as a critical guide for emergency responders to react appropriately to an accident. The cost of installing the software and training your staff is typically recovered within a surprisingly short period. For instance, a study of Accelrys CISPro inventory system calculated a potential $770,500 cost savings per year for a mid-sized research company with 50 employees and 10,000 chemicals. For your organization, this yearly savings could amount to $12,900 per laboratory employee and $12.50 per container of chemical inventory.
Read Also: What Is Hydraulic Lift In Physics
Transitioning To Digital Chemical Inventory Management
When you decide to implement chemical inventory software, be sure to choose a system that is right for your labs needs. It should be configurable, scalable, and intuitive enough that everyone in your lab, from assistants to the principal investigator, can learn to input and interpret data with minimal training. With a bar-code tracking system, a unique bar code adheres to every chemical container. When scanned, this bar code reveals the location, identity, quantity, and all safety information pertaining to the chemical. In addition, web-based chemical inventory systems provide access to all the inventory data you need at any time. The option to access your inventory information remotely, even when youre visiting a facility in another city, means more convenient, flexible, and worryfree monitoring of safety and inventory information.
Being a lab manager today is a more complex job than ever, requiring expert juggling of a gamut of responsibilities and taking on some personal liability for team safety and compliance. As your laboratorys research grows in scope and the size of your chemical inventory expands, make sure you can keep up by upgrading to a best-practices chemical inventory system.
Note: The full study referenced in this article is a part of Accelrys white paper titled Quantifying the Financial Benefits of Chemical Inventory Management Using CISPro.
Nomenclature Of Simple Compounds Using Stock Notation
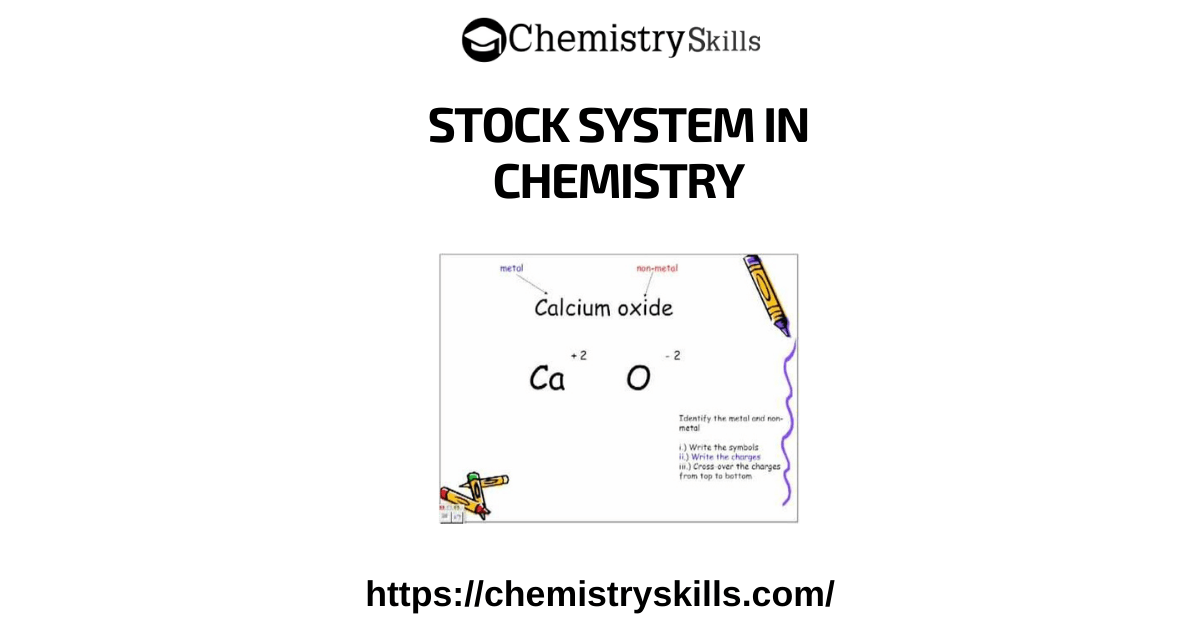
- Stock notation must be used on this page.
- Common names such as “ferrous” are not used.
- Spelling matters very much. No effort has been made to analyze input against possible spelling errors. There is a button below which can be pushed to display the spelling of all polyatomic ions used on this page.
- Capitalization does not matter, the page can adjust for improper capitalization.
Operation:
- Pressing “New Compound” displays a formula to the right of the table.
- Enter the name in the cell, breaking it into three parts and ensuring that there are parentheses around the Roman numeral.The Roman numeral should be contiguous with the metal, but your answer won’t be judged incorrect if there’s a break: ex- FeCl3 would be iron chloride.
- Press “Check Answer.”
- Results and score
- If you miss a problem three times, pressing “Show Answer” will display the complete solution and you will no longer be able submit an answer for that problem.
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
General Practices In Naming
The general practice among chemists is to use the more common chemical names whenever it is practical to do so, especially in spoken or informal written communication. Many of the common names are known and used mainly by the scientific community. Chemical substances that are employed in the home, the arts, or in industry have acquired traditional or popular names that are still in wide use. Many, like sal ammoniac mentioned above, have fascinating stories behind their names.
Sulfuric acid: The historical name for sulfuric acid is oil of vitriol. Medieval European alchemists prepared it by roasting green vitriol in an iron retort. Its chemical formula is .
Study More Efficiently Not Just More
- One of the first steps in coming up with an efficient study strategy is to assess what – in all of the things you are doing to study – seems to help you the most? What gave you the most confidence? If there are some things that you are already comfortable with, perhaps spend less time reviewing those and more time on concepts that are still challenging.
- Take some time to assess where are you having difficulty on the exams. When you get an exam back, retry all of the problems you missed . Do you get farther then you did during the exam? Are you really able to finish them with more time or in a less stressful environment? Do you get stuck on concepts or definitions? on math? on starting the problem?
- Debriefing the exam helps you indentify the conceptual gaps that you need to relearn versus errors that may have resulted from test stress or a misreading of a question.
- If you can start to identify where/how you are struggling with the exam, then you can think about how to make better use of your study time as you prepare for the next one.
Read Also: Hawkes Learning Systems Prealgebra And Introductory Algebra Answers
What Is Stock System Name
: a system in chemical nomenclature and notation of indicating the oxidation state of the significant element in a compound or ion by means of a Roman numeral that is used in parentheses after the name or part of the name designating this element and ending invariably in -ate in the case of an anion and that is placed
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Read Also: Who Are Paris Jackson’s Biological Parents
Compounds Composed Of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO2. Since these are different substances with different properties, they cannot both have the same name . To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element is first, followed by the name of the more nonmetallic element with its ending changed to the suffix ide. The numbers of atoms of each element are designated by the Greek prefixes shown in above in Table 5.
When only one atom of the first element is present, the prefix mono is usually deleted from that part. Thus, CO is named carbon monoxide, and CO2 is called carbon dioxide. When two vowels are adjacent, the a in the Greek prefix is usually dropped. Some other examples are shown in Table 6.
| Table 6. Names of Some Molecular Compounds Composed of Two Elements |
|---|
| Compound |