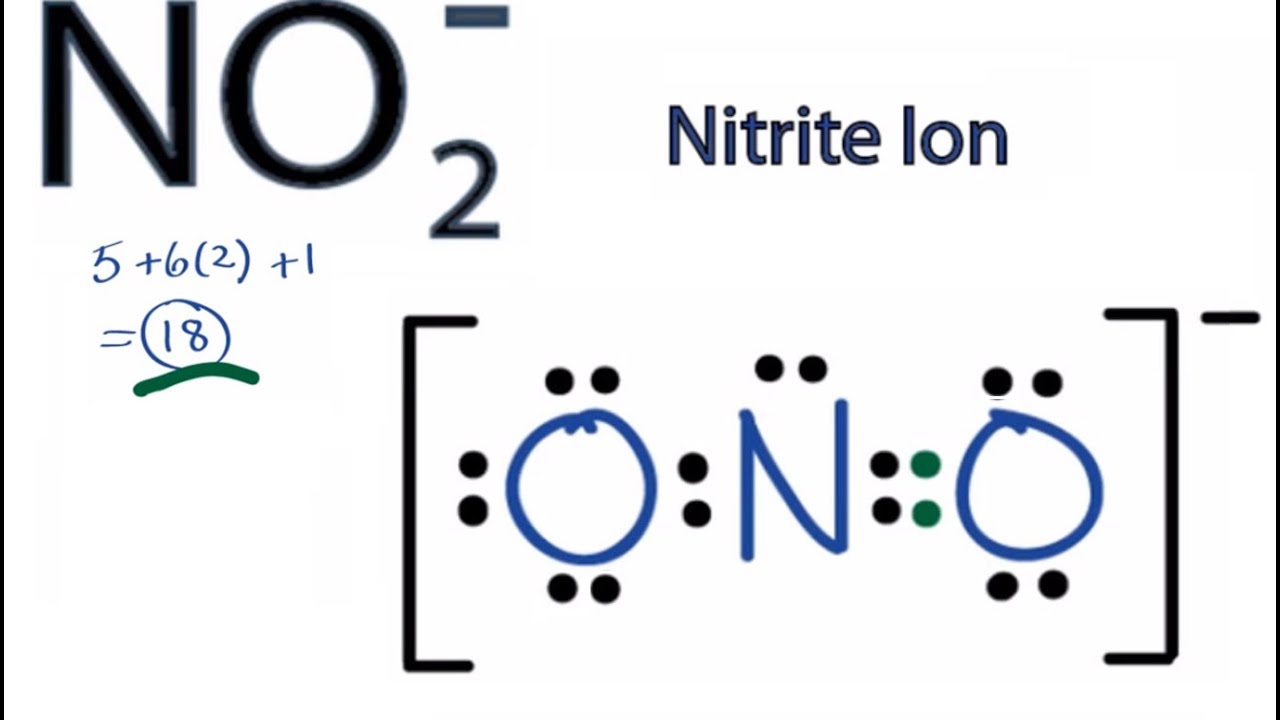
Total Number Of Electrons Of The Valance Shells Of Nitrogen And Oxygen Atoms And Charge Of The Anion
There are one nitrogen atom and two oxygen atoms in the nitrate ion. Also there is a -1 charge.
Nitrogen and oxygen are located at VA and VIA groups respectively in the periodic table. So nitrogen has five electrons in its valence shell. In oxygen atom, there are six electrons in its valence shell.
- Total valence electrons given by nitrogen atom = 5
There are two oxygen atoms in NO2, Therefore
- Total valence electrons given by oxygen atoms = 6 *2 = 12
Due to -1 charge, another electrons is added
- Due to -1 charge, received electrons = 1
- Total valence electrons = 5 + 12 + 1 = 18
What Makes No2 A Polar Molecule
Nitrogen has an electronegativity value of 3.04 and oxygen has that of 3.44.
This means there is an electronegativity difference between the two atomic elements. Although the difference is quite less, we have an asymmetrical bent molecular structure that induces the net dipole moment and makes NO2 a polar molecule in reality.
For detailed information, you should also once read out an article on the Polarity of NO2.
N2 Lewis Structure Molecular Geometry And Hybridization
Chemistry plays an essential role in the science world by showing the bond effect between the atoms of the molecules.
The atom is the most crucial part of a chemical element, breaking which we find protons, electrons, and neutrons. They all play a key member in the formation of chemical bonds.
Many scientists have incredibly contributed to different specialties of chemistry. One of them was American chemist, Gilbert N. Lewis who introduced the concept of electron dot structure in 1916.
The article the atom and the molecule tell about the position of valence shell electrons in a chemical bond. The concept is also commonly referred to as Lewis structures or simply Lewis dot structures.
Read Also: Who Is Generally Recognized As The Founder Of American Psychology
What Is The Structural Geometry Of The Nof Molecule
The electron dot diagram for NOF is as follows: The N atom has three electron groups on it, two of which are bonded to other atoms. The molecular shape is bent.
What is the Lewis structure for Nof?, The NOF Lewis structure is very similar to NOCl and NOBr. In the NOF Lewis structure Nitrogen is the least electronegative atom and goes in the center of the Lewis structure. Check the formal charges to be sure that each atom has a formal charge of zero.
Furthermore, What is the bond angle in the NOF molecule?, The bond angles are approximately 109.5° when all four substituents are the same. The NOF Lewis structure is very similar to NOCl and NOBr.
Finally, What is the molecular geometry of No?, Lewis Structures and the Shapes of Molecules
| Formula |
Determination Of Molecular Geometry

The shape of a molecule is determined by the bonded atom, although this does not mean the shape itself is unaffected by the presence of lone pair.
Molecular geometry includes geometrical parameters such as bond lengths, bond angles, and torsional angles that help determine the position of atoms as well as a molecules general shape.
It influences a substances properties such as its reactivity, color, polarity, magnetism, biological activity, and phase of matter.
There are various techniques to determine molecular geometry such as Raman spectroscopy, infrared spectroscopy, and microwave spectroscopy.
Read Also: Algebra Age Word Problems
Is No2 Diamagnetic In Nature
Nitrogen monoxide has 11 valence electrons, it is paramagnetic, with a single electron occupying the pair of orbitals. However, in the liquid and solid states, the unpaired electrons are involved in the formation of the loose dimer. In the absence of an unpaired electron, it is diamagnetic in nature.
What Is The Vsepr Theory
VSEPR Theory Definition: Valence Shell Electron Pair Repulsion theory is a phenomenon used in chemistry to predict the shapes of the individual molecules based on the repulsion acting between the electrons pairs in a molecule. The other name if VSPER theory is Gillespie- Nyholm theory, named after its two main developers.
Read Also: What Does Span Mean In Linear Algebra
Molecular Geometry Notation For No2+ Molecule :
Determine the form of NO2+ molecular geometry using VSEPR theory. The AXN technique is commonly used when the VSEPR theory is used to calculate the shape of the NO2+ molecule.
The AXN notation of NO2+ molecule is as follows:
The central nitrogen atom in the NO2+ molecule is denoted by the letter A.
The bound pairs of electrons to the core nitrogen atom are represented by X.
The lone pairs of electrons on the central nitrogen atom are denoted by the letter N.
Notation for NO2+ molecular geometry
We know that NO2+ is the core atom, with two electron pairs bound and zero lone pairs of electrons. The general molecular geometry formula for NO2+ is AX2.
According to the VSEPR theory, if the NO2+ molecule ion has an AX2 generic formula, the molecular geometry and electron geometry will both be linear forms.
| Name of Molecule | |
| The formal charge of NO2+on nitrogen | +1 |
The Influence Of Thermal Excitation
Since the motions of the atoms in a molecule are determined by quantum mechanics, “motion” must be defined in a quantum mechanical way. The overall quantum mechanical motions translation and rotation hardly change the geometry of the molecule. In addition to translation and rotation, a third type of motion is molecular vibration, which corresponds to internal motions of the atoms such as bond stretching and bond angle variation. The molecular vibrations are harmonic , and the atoms oscillate about their equilibrium positions, even at the absolute zero of temperature. At absolute zero all atoms are in their vibrational ground state and show zero point quantum mechanical motion, so that the wavefunction of a single vibrational mode is not a sharp peak, but an exponential of finite width . At higher temperatures the vibrational modes may be thermally excited , but they oscillate still around the recognizable geometry of the molecule.
To get a feeling for the probability that the vibration of molecule may be thermally excited,we inspect the Boltzmann factorβ â¡ exp, where ÎE is the excitation energy of the vibrational mode, k the Boltzmann constant and T the absolute temperature. At 298 K , typical values for the Boltzmann factor β are:
- β = 0.0890 for ÎE = 0500 cmâ1
- β = 0.0080 for ÎE = 1000 cmâ1
- β = 0.0007 for ÎE = 1500 cmâ1.
Also Check: Geometry Dash Mega Hack V5
Why Does Nitric Acid Turn Skin Yellow
Nitric acid is a corrosive acid and a powerful oxidizing agent. Concentrated nitric acid stains human skin yellow due to its reaction with the keratin. These yellow stains turn orange when neutralized. Systemic effects are unlikely, however, and the substance is not considered a carcinogen or mutagen.
Molecular Orbital Diagram Of N2
Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond.
According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
When two orbitals are added, the result is stable bonding molecular orbital and when orbitals are subtracted, it is called unstable anti-molecular bonding which has more energy than the latter one.
Considering the energy level diagram, the configuration of N2 is 1S2, *1S2, 2S2, *2S2, 2Px2, 2Py2, 2Pz1.
Also Check: Algebra 1 Eoc Fsa Practice Test Calculator Portion
Electron Geometry Vs Molecular Geometry
Geometry in chemistry refers to the shape of molecules in 3-Dimensional space.
Molecular geometry is described as the 3D arrangement of atoms in a molecule, normally relative to a single central atom. Whereas, electron geometry is the 3D arrangement of electron pairs around a central atom, whether bonding or non-bonding.
A lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond. And a bond pair is a pair of electrons in a bond.
It is well known that the electron pairs, being negatively charged, repel each other. This repulsion causes the electron pairs around the central atom to arrange as far apart from each other as possible. This minimizes the repulsion.
Under the influence of a single nucleus, a lone pair offers more repulsion than a bond pair which is influenced by two nuclei. This causes a slight decrease in bond angles .
If all of the electron groups are bond pairs , the molecular geometry and electron geometry are the same. An example is a methane molecule, CH4 with 4 bond pairs and no lone pairs, all 4 of carbons valence electrons are bonded with hydrogen atoms. Its molecular, as well as electronic geometry, is tetrahedral.
Prerequisite concept:
- Lewis Structure
- Valence Shell Electron Pair Repulsion Theory
What Is The Hybridization Of Nitrogen Dioxide
In the hybridization of nitrogen dioxide i.e. NO2 , let us take a look at the Nitrogen atom first. Here we will notice that the nitrogen atom is the centre atom and has only one lone electron. The atom, however, does not have an octet because it is short on electrons. Since there is an electron deficit in the nitrogen molecule, usually it tends to react with some other molecule for its octet completion. On the other hand, the two oxygen atoms have an octet of electrons each. When the bonding occurs, the two oxygen atoms will form a single and a double bond with the nitrogen atom.
At the same time, nitrogen must have three hybridized orbitals that are used to harbour the two sigma bonds and including one electron resulting in sp2 hybridization. The three sp2 hybrid orbitals present in nitrogen will have one electron, and the p orbital will also have one electron. However, when it forms two sigma bonds, only one p orbital and sp2 hybrid orbital will contain one electron each. The p orbital will then form a pi bond with the oxygen atom.
Read Also: Chapter 7 Quiz 1 Geometry
No2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram
Who has not heard about NO2, Nitrogen Dioxide?
It is one of the most common gaseous molecules having a reddish-brown hue. It can be cooled and compressed into a yellowish-brown liquid for tendshipping and transport.
A highly toxic poisonous chemical compound, NO2 is a major air pollutant and belongs to the group of oxides of nitrogen.
This chemical can be used for bleaching and sterilization purposes.
It has applications in tobacco and explosive industries. NO2 acts as an intermediate in HNO3 acid manufacturing as well as an oxidizer for fuels in rockets and space probes.
Below are the laboratory preparation methods of Nitrogen Dioxide.
Drawing The Lewis Structure For No2
Video: Drawing the Lewis Structure for NO2-
For the NO2- Lewis structure, calculate the total number of valence electrons for the NO2- molecule. After determining how many valence electrons there are in NO2-, place them around the central atom to complete the octets.
There are a total of 18 valence electrons for the Lewis structure for NO2-.
Nitrogen is the least electronegative atom in the NO2- lewis structure and therefore goes in the center of the structure.
In order to make sure the outer shell of the Nitrogen atom is full you will need to form a double bond with one of the Oxygen atoms in this Lewis structure.
Also note that you should put the NO2- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
It is helpful if you:
- Try to draw the NO2- Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to NO2- for more practice.
Read Also: Punchline Bridge To Algebra Worksheets
Rules To Draw Lewis Structure
- Firstly, check out the atomic number of each atom from the Periodic Table.
- Calculate the total number of valence electrons of the atoms present in a molecule.
- Take care of the octet rule where the ions or atoms should have eight electrons in their outermost valence shell
- While representing the bonds, you should know about lone and bonded pairs.
- Choose the central atom by identifying the least electronegative atom.
- Arrange the remaining electrons to the terminal atoms
Note: The most important thing about the Lewis dot structure is that only valence electrons take part in chemical bonding.
Lewis Structure Of No2
The Lewis structure of NO2 has 17 valence electrons. In a Lewis structure, it’s not common to have an odd number of valence electrons. For this reason, we’ll try to get closer to an octet as we can be on the central Nitrogen atom. It means it will only have 7 valence electrons.
The Nitrogen atom in the Lewis structure for NO2 is the least electronegative atom and passes at the centre of the structure.
You May Like: How To Solve Half Life Equations In Chemistry
Overview: No2+ Electron And Molecular Geometry
According to the VSEPR theory, the NO2+ molecule ion possesses linear molecular geometry. Because the center atom, nitrogen, has two N-O double bonds with the two oxygen atoms surrounding it. The O-N-O bond angle is 180 degrees in the linear NO2+ molecular geometry. The NO2+ molecule has a linear geometry shape because it contains two oxygen atoms in the linear and each of them have two lone pairs of electrons.
There are two N-O double bonds at the NO2+ molecular geometry. After linking the two oxygen atoms and no lone pairs of electrons on the nitrogen atom in the linear form, it maintains the linear-shaped structure. In the NO2+ molecular geometry, the N-O double bonds have stayed in the two terminals and no lone pairs of electrons on the nitrogen atom of the linear molecule.
The center nitrogen atom of NO2+ has no lone pairs of electrons, resulting in linear NO2+ electron geometry. However, the molecular geometry of NO2+ looks linear or cylinderical-shaped and has no lone pairs of electrons on the nitrogen of the NO2+ geometry. Itâs the NO2+ moleculeâs symmetrical geometry. As a result, the NO2+ molecule is nonpolar.
Difference Between Molecular Geometry And Electron Geometry
| Molecular geometry | Electron geometry |
| Molecular Geometry is the arrangement of atoms in a molecule, normally relative to a single central atom. | Electron Geometry is the arrangement of electron pairs around a central atom. |
| It excludes lone pairs in deciding the shape of a molecule, although repulsion from lone pair is taken into account only in bond angles. | It considers the presence of both bond pair and lone pair of electrons in determining the shape. |
Don’t Miss: Algebra 2 Trig Regents Reference Sheet
Properties Of Nitronium Ion
- The molar mass of NO2+ is 46.005 g·mol1.
- It is soluble in non-polar solvents and insoluble in polar solvents, The example of non-polar solvent is benzene, carbon tetrachloride, etc. and example, polar solvents is water, ammonia, etc.
- NO2+ gas is very toxic, and it can lead to human death.
- Because of the nitrogen atom has a +1 charge, it has a high electronic affinity and is used as an electrophoresis reagent in the titration process.
Summary
Above is the properties of NO2+.
Why Is No2+ An Electrophile

Here is the reason why NO2+ is as an electrophile, Electrophile has the species that are electron loving. Therefore, they usually have a positive charge and do not have an isolated pair. The Electrophile accepts the pair of electrons and forms a new covalent bond.
Summary
The Nucleophiles is different from electrophile because it provides a pair of electrons to form a chemical bond associated with the reaction.
Recommended Reading: How To Intimidate Someone Psychologically
Some Key Points To Remember
A few basic key points that are to remember are listed below.
-
The two oxygen atoms have each electrons octet
-
In nitrogen dioxide , there are 1 lone electron pair and 2 sigma bonds
-
The p orbital of nitrogen atom forms a pi bond with the oxygen atom
1. Which Bond Angle is Larger Among NO2+ and NO2- ?
In NO2, that is, in the nitronium ion, the N-atom has sp-hybridization thus, it adopts the linear geometry, and the O-N-O bond angle is 180°.
While coming to NO2, that is, nitrite ion, the N-atom has sp2hybridization thus, it adopts the bent geometry, for NO2, and the actual O-N-O bond angle is 115 ° .
This is the ‘nitronium ion,’ and it clearly shows that the ONO bond angle in NO2 is 180°.
And, this is the geometry or structure of NO2, having 115 NON bond angle.
So, the NO2-has a bond angle less than NO2+.
2. Why Does the NO2Carry a Negative Charge?
As that set of atoms are more stable in that configuration while an additional electron is available, the octet rule clarifies that an atom is more stable if it has a filled outermost orbital of s and p. There are one s, and 3 ps, and each orbital carries 2 electrons, so, as a result, eight is the good number.
Electronic Structure And Geometry Of No2+ And No2
51 ab initio
REFERENCESSection:
Recommended Reading: Chemistry Redken Treatment