Calculating Lone Pairs Of Electrons In Ch4 Molecular Geometry:
1.Determine the amount of lone pairs on the core carbon atom of the CH4 Lewis structure.Because the lone pairs on carbon are mostly responsible for the CH4 molecule geometry distortion, we need to calculate out how many there are on the central carbon atom of the Lewis structure.
Use the formula below to find the lone pair on the CH4 molecules central carbon atom.
L.P = V.E N.A/2
Lone pair on the central carbon atom = L.P
The core central carbon atoms valence electron = V.E
Number of C-H bonds = N.A
calculation for carbon atom lone pair in CH4 molecule
In the instance of CH4, the central atom, carbon, has four electrons in its outermost valence shell and four C-H bond connections.
As a result of this, L.P = /2=0
In the CH4 electron geometry structure, the lone pair on the central carbon atom is zero. It means there are no lone pairs in the core carbon atom.
Is Ch4 Polar Or Nonpolar
CH4 is a nonpolar molecule having a symmetric tetrahedral geometrical structure and four identical C-H bonds. Carbon and hydrogen have electronegativity values of 2.55 and 2.2, respectively, resulting in an equal distribution of electrons between carbon and hydrogen. Methane is a colorless, odorless, and highly flammable gas that is used to create energy and heat homes worldwide. It is used in chemical processes to produce other important gases, including hydrogen and carbon monoxide, as well as carbon black, a chemical component found in some types of rubber used in vehicle tires.
| Name of molecule |
- The molar mass of CH4 is 16.04 g/mol.
- In the CH4 lewis structure, there is a single bond between carbon and hydrogen atoms
Whats The Electron Pair Geometry For Ch4
Why is the geometry of NH4+ ion tetrahedral?
In NH4+ion four bond pairs are present. The repulsion between bond pairs is less as compared to the bond pair-lone pair repulsion. Therefore the geometry is tetrahedral. Early symptoms of spinal muscular atrophy may surprise you. Signs of spinal muscular atrophy can be easily ignored. Look for spinal muscular atrophy symptoms.
You May Like: What Does Quantum Physics Say About Reality
The Geometrical Structure Of Methane
The single-molecule of methane is tetrahedral with no lone pairs on any atom. This behavior is explained with the help of the Valence Shell Electron Pair Repulsion theory.
This theory is used to predict the geometrical structure of a molecule along with the reason for such a shape.
For the methane molecule, this theory says as there exists no distortion in the structure of CH4, it is an ideal bent-shaped molecule or tetrahedron having a bond angle of 109.5° between hydrogen-carbon-hydrogen atoms .
Due to the symmetrical shape of the bonds formed in the CH4 molecule, the charges on its atoms are equally distributed and no polarization takes place ie the Methane molecule is a nonpolar molecule.
For better understanding, you can refer to the article written on the polarity of CH4.
The distortion from the ideal bond angle within a molecule occurs because of the presence of lone pairs and bond length between the central atom and the side atoms.
From the Lewis structure, it can be understood that an equal number of electron sharing is taking place between the carbon atom and four hydrogen atoms altogether.
It is the reason why the structure of methane is highly stable in nature.
How To Give Notation For Ch4 Molecule:
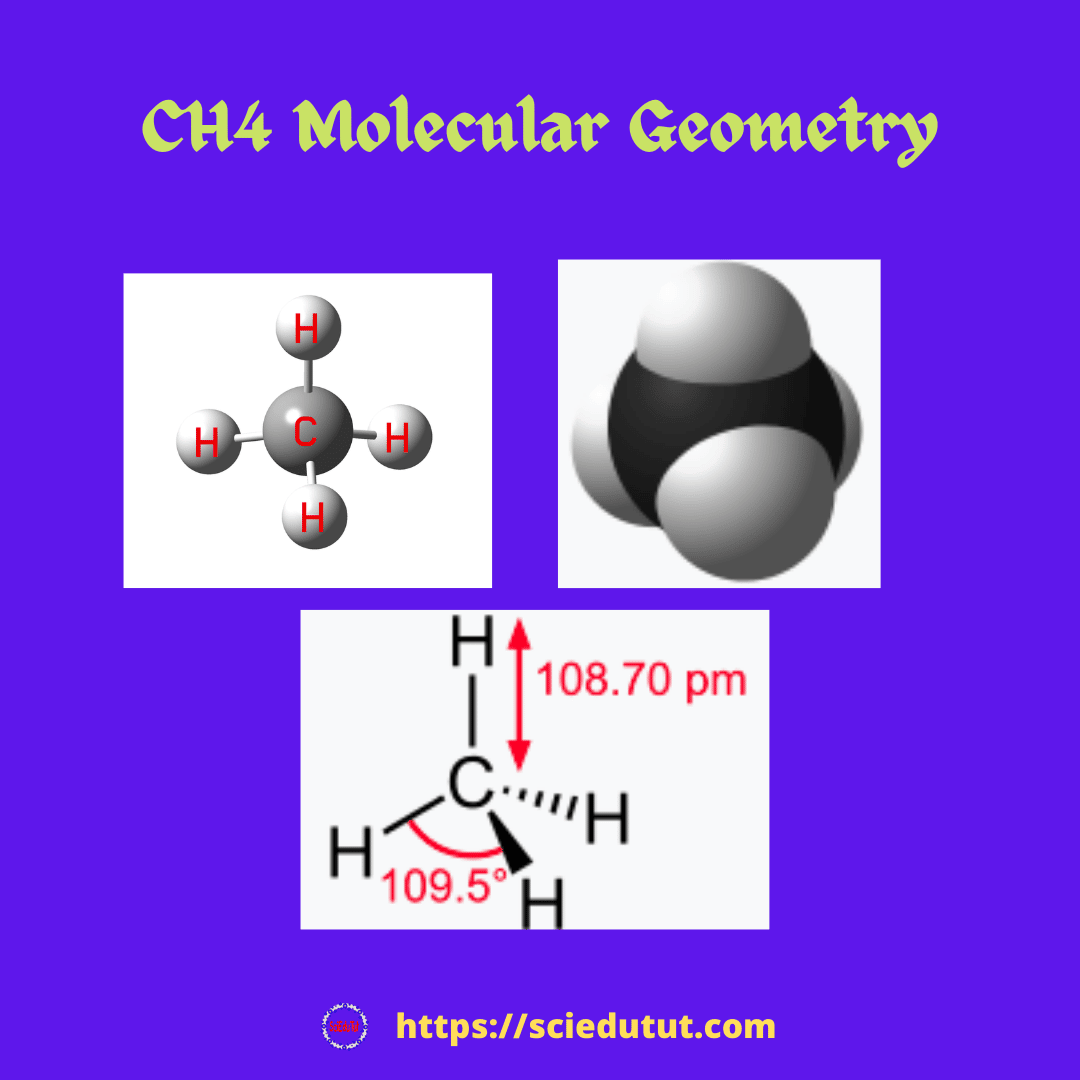
Determine the form of CH4 molecular geometry using VSEPR theory. The AXN technique is commonly used when the VSEPR theory is used to calculate the shape of the CH4 molecule.
The AXN notation is as follows:
The center carbon atom in the CH4 molecule is denoted by the letter A.
The bound pairs of electrons to the core atom are represented by X.
The lone pairs of electrons on the center carbon atom are denoted by the letter N.
Notation for CH4 molecular geometry
We know that carbon is the core atom, with four electron pairs bound and zero lone pairs. due to the Lewis structure of CH4 The general molecular geometry formula for CH4 is AX4.
According to the VSEPR theory, if the molecule has an AX4 generic formula, the molecular geometry and electron geometry will both be tetrahedral.
| Name of Molecule |
You May Like: How To Analyze People With Psychology
Electron Geometry Vs Molecular Geometry
Electron geometry and molecular geometry are the arrangements of electrons or atoms in three-dimensional space around a central atom. This gives a molecule a specific shape and bond angles.
Electron geometry helps us in determining the arrangement of various electron groups. Molecular geometry, on the other hand, helps us in determining the entire atom and its configuration. It is the three-dimensional arrangement of all the atoms in a given molecule.
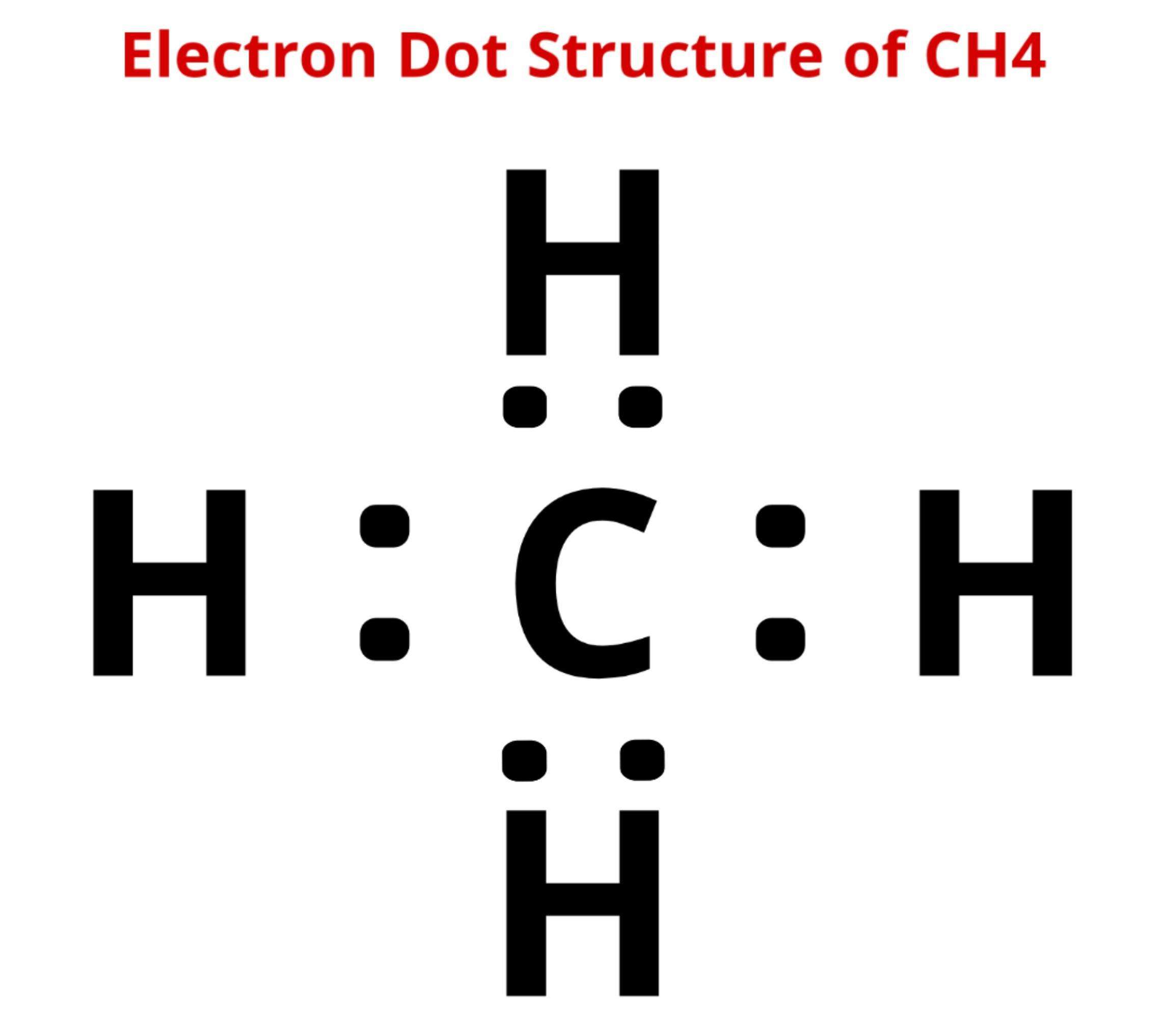
Lewis Structure Of Ch4
The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons.
Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.
Firstly, look for the total number of valence electrons required by a single CH4 molecule, which is sixteen.
Next, a search of electrons is required by a single CH4 molecule to reach a stable condition.
It is eight for a single CH4 molecule, as four are needed by the carbon atom and one by hydrogen atom each.
The next step is to find the total number and type of bond-forming that atoms within a single CH4 molecule.
A single shared covalent bond is formed between each carbon and hydrogen atom .
Lastly, search for the central atom that is usually the single atom in a molecule. It is carbon in the case of methane .
Now, draw the lewis structure of the methane as below
Read Also: What Do You Learn In 4th Grade Math
What Is The Geometry Of The Methane Molecule
The simplest hydrocarbon , methane is a gas with a chemical formula of CH4 and a molecular weight of 16.04.
To Rotate the Molecule—> Left Click and Drag
To Zoom--> > Left Click + hold Shift button and Drag Vertically
Jsmol Menu —> > Right-Click
Style –> Schemes –> wireframe
Label atoms:
Style –> Label —> atom number
To measure angles: hold left mouse bottom over atom and double click on atom 1, drag to second atom single click center atom, drag and double click atom 3.
Measure the following angles:
Below is shown the molecule Methane.
What polyhedral stucture would you think the methane molecule best represents? View the flash video below to see…
About Methane:
Methane is a principal component of natural gas. When a single molecule of methane is burned in the presence of oxygen releases one molecule of CO2[carbon dioxide) and two molecules of H2O .
CH4 + 2O2 —> CO2 + 2H2O
The strength of the carbon hydrogen covalent bond in methane is among the strongest in all hydrocarbons.
Pure methane is odorless, but when used as a fuel it is usually mixed with small quantities of strongly-smelling sulfur compounds such as ethyl mercaptan to enable the detection of leaks.
Methane is a greenhouse gas with a global warming potential of 22 . Methane results from the decomposition of certain organic matters in the absence of oxygen. It is therefore also classified as a biogas.
Why Does Ammonia Have A Tetrahedral Electron Geometry But A Trigonal Pyramidal Molecular Geometry
Three of these electron pairs are used as bond pairs which leaves one lone pair of electrons. The lone pair repel more strongly than bond pairs giving tetrahedral arrangement. Since the lone pairs are invincible the shape of ammonia is trigonal pyramidal.
Why does CH4 have a tetrahedral geometry?
We now rationalize the tetrahedral arrangement of atoms around methane as being due to the repulsion of the bonding pairs of electrons with each other .
Don’t Miss: What Are The Possible Benefits Of Studying Biology
Stability Of The Periodic Table Trend In Group 17 Elements
One trend in Group 17 elements that stand out among all others is that as you go down a group to a minor feature, regardless of whether it is a halogen or not. This trend can be seen when looking at radii or first ionization energy.
This could be due to an inert pair effect making these elements more stable than those with no idle pairs . With that being said, two exceptions are ICl which has a larger ionic radius than IBr and ICl, which has lower first ionization energy than Br or Cl. These two exceptions can be explained by reasons different from an inert pair effect.
Physical Properties Of Group 17 Elements
All of these elements are gases under standard conditions. All have relatively large atoms for example, bromine has a radius larger than iodines . The period 17 elements all have octet electron configurations except bromine which has seven electrons in its valence shell so it can form diatomic molecules as well as polyatomic ions with various stoichiometries: HBr · HBr · HI · BrCl · BFF 3, etc.
Read Also: What Was The Geography In The New England Colonies
What Is The Molecular Geometry Of H2co
According to VSEPR, the lowest energy can be obtained by minimizing repulsion between electron pairs around the central atom, resulting in the most stable geometry.
Throughout formalin, we will look at the electron pairs surrounding Carbon. We need the steric number of Carbon, which is the number of atoms bonded toward the nitrogen carbon depending on the number of lone pair of electrons on the central atom, to apply VSEPR. It is three for Carbon.
The molecular shape seems tetragonal flat when whole realms = three and lone pair = 0.
The molecular geometry of hydrogen chloride is considered to be in the shape of a trigonal pyramidal shape. The HCl molecule comprises one H atom bonded to one Cl atom. The bond length between the two atoms is about 154 pm. Bond angles are about 90°. The H and Cl atoms are held together by two covalent bonds within a molecular orbital.
The HCl molecule is held together by two strong, covalent bonds. The bond length between the two atoms is 154 pm. Bond angles are about 90°. The HCl molecule is held together by two covalent bonds within a molecular orbital. The H and Cl atoms are held together by two covalent bonds. The bond length between the two atoms is 154 pm. Bond angles are about 90°.
How To Draw Lewiss Structure Of Oxygen
In the O2 Lewis structure, there is a double bond between two oxygen atoms.Oxygen is a diatomic nonpolar molecule with a bond angle of 180 degrees.In its molecule, both oxygen atoms have the same electronegativity value, and both atoms share equal ratios of bonded shared electrons, so the overall O2 molecule turns out to be nonpolar in nature.
Read Also: Algebra 1 Chapter 2 Test
Overview: Ch4 Electron And Molecular Geometry
According to the VSEPR theory, CH4 possesses a tetrahedral molecular geometry and a CH4-like electron geometry. Because the centre atom, carbon, has four C-H bonds with the four hydrogen atoms surrounding it. The H-C-H bond generates a 109-degree angle in tetrahedral geometry. The CH4 molecule has a tetrahedral shape because it contains four hydrogen atoms.
There are four C-H bonds at the top of the tetrahedral geometry. After linking the four hydrogens in the tetrahedral form, it maintains the tetrahedral-like structure. In the CH4 tetrahedral geometry, the C-H bonds are enclosed.
The centre carbon atom of CH4 has no lone pairs of electrons, resulting in tetrahedral electron geometry. However, the molecular geometry of CH4 is tetrahedral in nature. Its the CH4 molecules asymmetrical geometry. As a result, the CH4 molecule is nonpolar.
The Bond Angle Of Ch4
As we know the molecular geometry of CH4 is regular tetrahedral with no distortion, hence, according to the VSEPR theory, for a regular tetrahedral structure, the bonded atoms around the central atom will spread at an angle of approx 109.5° to minimize the repulsion and attains stability.
Hence,the bond angle ofHCH in CH4 is 109.5°.
You May Like: What Does Relativity Mean In Physics
Is Bf3 Polar Or Nonpolar
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out. Even if each B-F bond is polar, the net dipole moment is zero because adding the bond vectors cancels everything out.Check out the full article Is BF3 polar or nonpolar?.
Why Methane Is Nonpolar
Natural gass primary component, methane, is a nonpolar molecule. In a three-dimensional configuration fashioned like a four-sided pyramid, four hydrogen atoms encircle a single carbon atom. The symmetry of the hydrogens on the pyramids corners distributes electric charge uniformly across the molecule, making it nonpolar.
Don’t Miss: What Does Fn Mean In Physics
What Is The Hybridization Of Methane
When we talk about CH4 it is basically a combination of 1 carbon and 4 hydrogen atoms. However, to form this compound the central atom carbon which has 4 valence electrons obtain more electrons from 4 hydrogen atoms to complete its octet. When the electrons are shared between carbon and hydrogen there is a formation of a covalent bond or bonds to be more accurate.
Now coming to the hybridization of methane, the central atom carbon is sp3 hybridized. This is because one 2s orbital and three 2p orbitals in the valence shell of carbon combine to form four sp3 hybrid orbitals which are of equal energy and shape. Further, four H atoms also use these four sp3 hybrid orbitals of carbon to form C-H sigma bonds which ultimately leads to the formation of the methane molecule.
Ch4 Lewis Structure Hybridization Molecular Geometry Bond Angle And Shape
Methane is one of the simple organic molecules, given its straightforward structure. It has the chemical formula of CH4 and comprises one carbon atom forming bonds with four hydrogen atoms. The compound is one of the main constituents of natural gas. It is also known as an alkane. Talking about its properties, Methane is a colorless and flammable gas. It is formed by the decaying of natural minerals and is widely used as fuel. CH4 is also used in the natural production of several organic compounds.
| Name of molecule | |
| No of Valence Electrons in the molecule | 8 |
| Molecular Geometry of CH4 | Tetrahedral |
In this blog post, we will find the Lewis Structure, Molecular Geometry, and Shape of the molecule. But before proceeding with the Lewis Dot Structure, we will first look at the total number of valence electrons for this molecule as these are the ones that participate in the bond formation.
Contents
Don’t Miss: What Is Alt Sgpt In Blood Chemistry
Why Is Nh3 Tetrahedral
Ammonia has 4 regions of electron density around the central nitrogen atom . These are arranged in a tetrahedral shape. The resulting molecular shape is trigonal pyramidal with H-N-H angles of 106.7°.
Why is NH3 pyramidal instead of trigonal planar?
Ammonia is Trigonal Pyramidal because the lone pair of electrons on ammonia repels the pair of electrons in the 2 N-H bonds. Ammonium ion, as we know, is NH4+. Once that lone pair of electrons attacks a hydrogen ion, the structure of the molecule becomes Tetrahedral.
Does CH4 have a pyramidal shape?
Four electron pairs arrange themselves in space in what is called tetrahedral arrangement. A tetrahedron is a regular triangularly-based pyramid.What is the shape of CH4? Pyramidal. Trigonal planar. Linear. Tetrahedral.
| tetrahedral geometry for the four shape-determining electron pairs |
What is the electron geometry of CH4?
The molecular geometry of CH4 is tetrahedral and its electron geometry is also tetrahedral because as per VSEPR theory, molecular shape considers only bond pairs or atoms while electron geometry considers bonded atoms as well as lone pairs present on the central atom.
Is Sicl4 Polar Or Nonpolar
SiCl4 is a nonpolar molecule. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity between the anion and the cation is between 0.4 and 1.7, such bonds occur.Check the full article Is SiCl4 polar or nonpolar?.
Recommended Reading: What Is Single Replacement In Chemistry
Tetrahedral Molecules With No Central Atom
A few molecules have a tetrahedral geometry with no central atom. An inorganic example is tetraphosphorus which has four phosphorus atoms at the vertices of a tetrahedron and each bonded to the other three. An organic example is tetrahedrane with four carbon atoms each bonded to one hydrogen and the other three carbons. In this case the theoretical CCC bond angle is just 60° , representing a large degree of strain.
Calculate The Number Of Molecular Hybridizations Of Ch4 Molecule
How do you find the CH4 molecules hybridization? We must now determine the molecular hybridization number of CH4.
The formula of CH4 molecular hybridization is as follows:
No. Hyb of CH4 = N.A + L.P
No. Hy of CH4= the number of hybridizations of CH4
Number of C-H bonds = N.A
Lone pair on the central carbon atom = L.P
Calculation for hybridization number for CH4 molecule
In the CH4 molecule, carbon is a core atom with four hydrogen atoms connected to it and no lone pairs. The number of CH4 hybridizations can then be estimated using the formula below.
No. Hyb of CH4= 4+0 =4
The CH4 molecule has four hybridization sites. The sp3 hybridization is formed when one S orbital and three p orbitals join.
Don’t Miss: Houghton Mifflin Harcourt Publishing Company Algebra 2 Answer Key
Ch4 Molecular Geometry And Bond Angles
We have already discussed the bond formation and hybridization process above. Determining CH4 molecular geometry should be easier. In methane, the four hybrid orbitals are located in such a manner so as to decrease the force of repulsion between them. Nonetheless, the four orbitals do repel each other and get placed at the corners of a tetrahedron. CH4 has a tetrahedral shape. The sp3 hybrid orbitals have a bond angle of 109.5o.