What Are 3 Ways To Measure The Concentration Of A Solution
What are 3 ways to measure the concentration of a solution? Concentration is the measure of how much solute is mixed with the solvent. The 3 ways to measure the concentration are
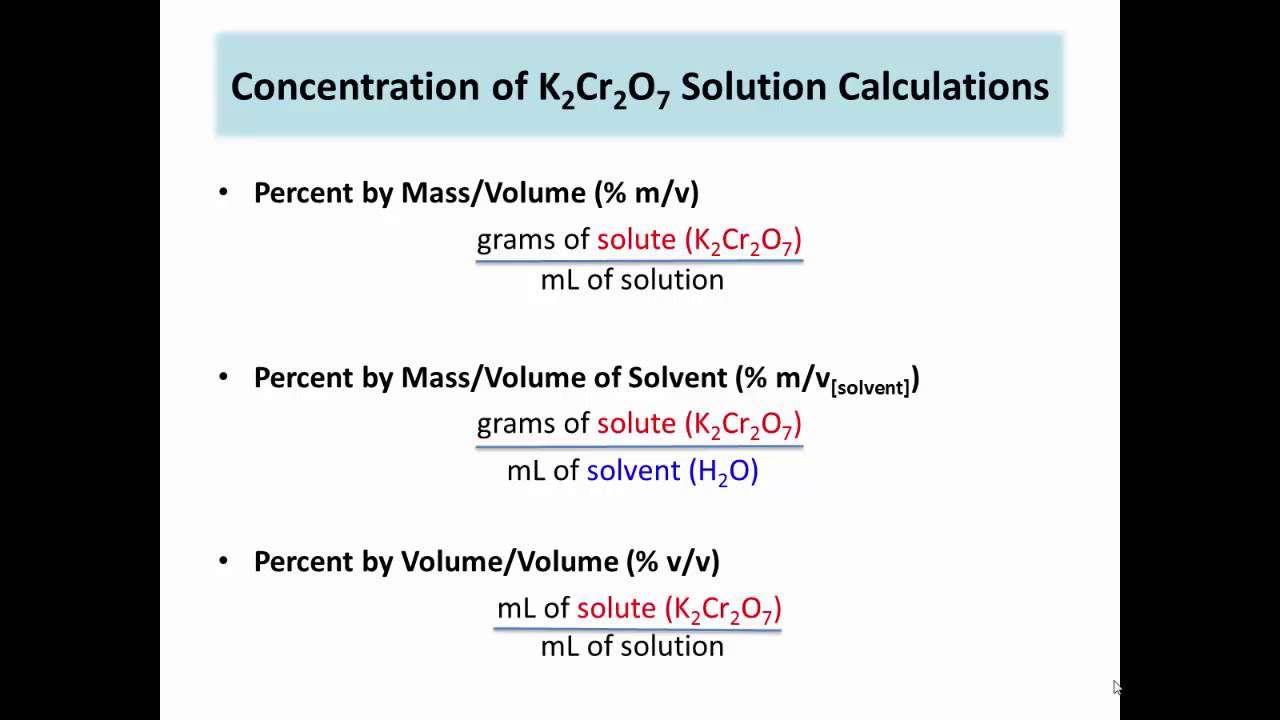
- Using the standard formula C= m/v
Here, C is the concentration
m is the mass of the solute dissolved
V is the volume of the solution
- The concentration of the solution can be found in percentage by multiplying the concentration value by 100.
- The molar concentration or molarity can be calculated using the following formula
M = mol/V
M is the molarity of the solution
mol is the number of moles of the solute
V is the volume of the solvent.
Was this answer helpful?
What Is Meant By Concentration Of A Solution
In an aqueous solution, two parts exist, namely solute and solvent. They are the two basic solution concentration terms that you need to know. We always need to keep an account of the amount of solute in the solution. The amount of solute in the solvent is what is called the concentration of a solution. In chemistry, we define concentration of solution as the amount of solute in a solvent. When a solution has more solute in it, we call it a concentrated solution. Whereas when the solution has more solvent in it, we call it a dilute solution. Now that you understand the concept of what is concentration of solution let’s move on to the different methods of expressing concentration.
The image shows a solution from the most dilute solution to the most concentrated solution.
More Ways To Calculate And Express Concentration
There are other easy ways to express the concentration of a chemical solution. Parts per million and parts per billion are used primarily for extremely dilute solutions.
g/L= grams per liter = mass of solute / volume of solution
F= formality = formula weight units per liter of solution
ppm = parts per million = ratio of parts of solute per 1 million parts of the solution
ppb= parts per billion = ratio of parts of solute per 1 billion parts of the solution.
Recommended Reading: Holt Geometry Practice Workbook Answer Key
Chemistry End Of Chapter Exercises
0.444 mol of CoCl2 in 0.654 L of solution
98.0 g of phosphoric acid, H3PO4, in 1.00 L of solution
0.2074 g of calcium hydroxide, Ca2, in 40.00 mL of solution
10.5 kg of Na2SO4·10H2O in 18.60 L of solution
7.0 Ã 10â3 mol of I2 in 100.0 mL of solution
1.8 Ã 104 mg of HCl in 0.075 L of solution
1.457 mol KCl in 1.500 L of solution
0.515 g of H2SO4 in 1.00 L of solution
20.54 g of Al3 in 1575 mL of solution
2.76 kg of CuSO4·5H2O in 1.45 L of solution
0.005653 mol of Br2 in 10.00 mL of solution
0.000889 g of glycine, C2H5NO2, in 1.05 mL of solution
Outline the steps necessary to answer the question.
Answer the question.
Outline the steps necessary to answer the question.
Answer the question.
Key Concepts And Summary
Solutions are homogeneous mixtures. Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.
Read Also: Bond Geometry Chart
Calculating Moles Of Solute
Calculate the moles of copper sulfate in 250.00 mL of 0.020 mol L-1 copper sulfate solution.
n = moles of solute = ? mol
Extract the data from the question: c = concentration of solution = 0.020 mol L-1 V = volume of solution = 250.00 mL = 250.00 ÷ 1000 = 250.00 x 10-3 L = 0.25 L
Write the equation:
n = 0.020 × 0.25 = 0.0050 mol
Calculating Molality Given Mass
If we mass 5.36 g of KCl and dissolve this solid in 56 mL of water, what is the molality of the solution? Remember that molality is moles of solute/kg per solvent. KCl is our solute, while water is our solvent. We will first need to calculate the amount of moles present in 5.36 g of KCl:
\text = 5.36 \text \times = 0.0719 \text
We also need to convert the the 56.0 mL of water to its equivalent mass in grams by using the known density of water :
56.0\ \text \times = 56.0\ \text
56.0 g of water is equivalent to 0.056 kg of water. With this information, we can divide the moles of solute by the kg of solvent to find the molality of the solution:
\text = = = 1.3\ \text
The molality of our KCl and water solution is 1.3 m. Since the solution is very dilute, the molality is almost identical to the molarity of the solution, which is 1.3 M.
Don’t Miss: 8th Grade Algebra Word Problems
Measuring Concentrations Of Solutions
- Differentiate between solute, solvent, and solution
- Calculate the molar concentration for solutes
- Outline the steps to make a solution of a desired concentration from a solid or aqueous solute
- Calculate the concentration of ions in a soluble ionic compound
- Perform stoichiometric calculations involving aqueous solutes
- Calculate the concentration of unknown solutes
Concentration Of A Solute
There are two basic ways of reporting the concentration of a solute in a solvent, by reporting the mass of solute in a given volume, or the number of moles of solute in a given volume. These are effectively conversion factors that define the equivalent mass or moles of a solute to the volume of the solution.
- Mass Concentration: has typical units of g/L \
- Mole Concentration : has units of mol/L or M \
So a 3.0M solution of sucrose has 3.0 mole of sucrose per liter
How do we convert between mass and mole based concentration?
Simply by multiplying or dividing by the molar mass . If you know the g/L, you simply divide by the molar mass, and if you know the moles/L and want the mass/L, you multiply by the molar mass .
How do we “count” solute molecules and ions?
This can be contrasted to how we “count” chemical entities that are pure solids.
Making a Solution with a Solid Solute.
Step 1: Calculate Mass of solute needed for desired volumeStep 2: Quantitatively Transfer Mass to Volumetric FlaskStep 3: Dilute to volume with solvent, ensuring that all of the solid has dissolved.
Making 500.0 mL of 0.500 M CopperSulfate
Step 1: Calculate Mass CuSO4 needed.
Step 2: Weight 39.9g CuSO4 and quantitatively transfer to 500 mL graduated cylinder , being sure all the salt is transferred.
Step 3: Fill half way and mix, then dilute to volume. Make sure all the solute is dissolved, and recheck that solution level is at bottom of meniscus.
Exercise \
- Answer
-
1.57 M C6H12O6
Exercise \
Read Also: Who Are Paris Jackson’s Biological Parents
How To Calculate Molality Of A Solution
Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same.
Calculate Molality: moles solute per kilogram solvent
symbol: m
m = moles / kilogram
Example: What is the molality of a solution of 3 grams of KCl in 250 ml of water?
First, determine how many moles are present in 3 grams of KCl. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. Then add them together to get the grams per mole for KCl.
- K = 39.1 g/mol
- KCl = 39.1 + 35.5 = 74.6 g/mol
For 3 grams of KCl, the number of moles is:
* 3 grams = 3 / 74.6 = 0.040 moles
Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water , but you also have 3 grams of solute, so the total mass of the solution is closer to 253 grams than 250. Using 2 significant figures, it’s the same thing. If you have more precise measurements, don’t forget to include the mass of solute in your calculation!
- 250 g = 0.25 kg
- m = 0.040 moles / 0.25 kg = 0.16 m KCl
Finding Concentration In Percentage Or Parts Per Million
Read Also: How To Find Delta Math Answers With Inspect Element
Using The Mass Per Volume Equation
Tip: If you need to use a scale, subtract the mass of the container youre using to hold the solute or else your calculations will be off.
What Is Concentration In Chemistry Examples
concentrationExample
. Consequently, what is an example of concentration?
The definition of concentration means the amount of ingredients or parts in relation to the other ingredients or parts. An example of concentration is the amount of salt to water in a saltwater solution. An example of concentration is a student focusing all of her after school time on a specific research paper.
Also, what is an example of a concentrated solution? Common commercial examples of concentrated solutions are hydrochloric acid and sulfuric acid. Hand soap, soft drinks and liquid medicine are concentrated solutions commonly found in the household. Concentrated solutions are best understood relative to dilute solutions. Tap water is an example of dilute solution.
Beside this, what is concentration in chemistry?
Concentration Definition. In chemistry, concentration refers to the amount of a substance in a defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution. However, the solute concentration may also be expressed in moles or units of volume.
What is the unit of concentration?
Molarity indicates the number of moles of solute per liter of solution and is one of the most common units used to measure the concentration of a solution. Molarity can be used to calculate the volume of solvent or the amount of solute.
Don’t Miss: Holt Mcdougal Worksheets
How To Find The Concentration Of A Solution Using Different Methods
There are various methods of expressing concentration of a solution. You will usually see Chemists working with the number of moles. Pharmacists will use percentage concentrations instead of the number of moles. Hence it is important to understand all the methods of expressing concentration of solutions.
The concentration of solution formula is given as follows.
Cor S = \
We will also see other methods on how to calculate concentration of a solution based on the different methods of expressing concentrations.
Concentration Units For Solutions
Chemistry is a science which deals a lot with solutions and mixtures. Knowing just how much of one thing is mixed in with a solution is an important thing to know. Chemists measure this by determining the concentration of the solution or mixture.
There are three terms that need to be defined in concentration discussions: solute, solvent and solution.
Solute: The dissolved substance added to the solution.Solvent: The liquid that dissolves the solute.Solution: The combination of solute and solvent.
The relationship between these three terms is expressed by many different concentration units. The unit you choose to use depends on how the solution is going to be used in your experiments. Common units include molarity, molality, and normality. Others are mass percent, mole fraction, formality and volume percent. Each unit is explained along with information about when to use them and the formulas needed to calculate the unit.
Molarity
Molarity is the most common concentration unit. It is a measure of the number of moles of solute in one liter of solution. Molarity measurements are denoted by the capital letter M with units of moles/Liter.
The formula for molarity is
This shows the number of moles of solute dissolved in a liquid to make one liter of solution. Note the amount of solvent is unknown, just that you end up with a known volume of solution.
A 1 M solution will have one mole of solute per liter of solution. 100 mL will have 0.1 moles, 2L will have 2 moles, etc.
Molality
Don’t Miss: Linear Algebra Span Definition
Calculating Volume Given Molarity And Moles
We can also calculate the volume required to meet a specific mass in grams given the molarity of the solution. This is useful with particular solutes that cannot be easily massed with a balance. For example, diborane is a useful reactant in organic synthesis, but is also highly toxic and flammable. Diborane is safer to use and transport if dissolved in tetrahydrofuran .
How many milliliters of a 3.0 M solution of BH3-THF are required to receive 4.0 g of BH3?
First we must convert grams of BH3 to moles by dividing the mass by the molecular weight.
\frac\text_3 }\text_3} = 0.29 \text\text_3
Once we know we need to achieve 0.29 moles of BH3, we can use this and the given molarity to calculate the volume needed to reach 4.0 g.
\text_}=\frac_}}}
3.0 \text = \frac_3} }
\text = 0.1 \text
Now that we know that there are 4.0 g of BH3 present in 0.1 L, we know that we need 100 mL of solution to obtain 4.0 g of BH3.
Solutions Of Solids In Liquids
Don’t Miss: Segment Addition Postulate Color By Number Worksheet Answer Key