Classification Of Organic Compounds
Organic compounds can be classified into:
- aromatic hydrocarbons . They are cyclic and stable organic compounds, which have alternating single and double carbon-carbon bonds in their structure.
- Aliphatic hydrocarbons . They are simple non-aromatic hydrocarbons , that is, they have a structure in the form of a linear or cyclic chain, but without alternating double and single bonds between their carbon atoms .
- heterocyclic . They are organic compounds in whose cyclic structure one or more of its carbon atoms has been replaced by atoms of other elements, such as nitrogen, sulfur or oxygen.
- Organometallics . They are organic compounds whose carbon atoms are covalently bonded to a metal atom.
- polymers . They are large macromolecular chains made up of smaller units and linked together by covalent bonds.
What Is A Matter In Chemistry
The term matter refers to anything that occupies space and has massin other words, the stuff that the universe is made of. All matter is made up of substances called elements, which have specific chemical and physical properties and cannot be broken down into other substances through ordinary chemical reactions.
What Is A Chemical Property
A chemical property is a characteristic of a particular substance that can be observed in a chemical reaction. Some major chemical properties include flammability, toxicity, heat of combustion, pH value, rate of radioactive decay, and chemical stability.
A chemical change or reaction is a process in which one substance changes to another substance. In this process, the characteristics of the substances change, and this is when chemical properties are observed.
A chemical property is not to be confused with a physical property, which includes such characteristics as shape , color, texture, flexibility, density, and mass.
Read Also: Toe 2 Geometry Dash
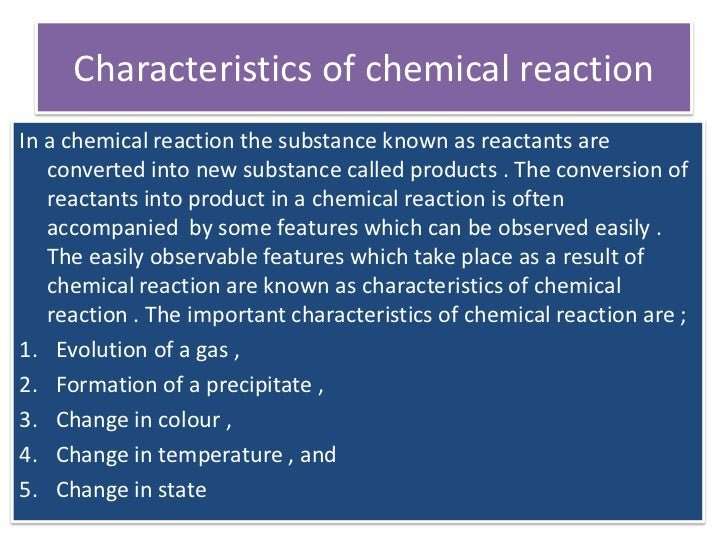
State Three Characteristics That Show A Chemical Reaction Has Occurred
Get Answer to any question, just click a photo and upload the photo and get the answer completely free,
UPLOAD PHOTO AND GET THE ANSWER NOW!
Answer
Get Link in SMS to Download The Video
Aap ko kya acha nahi laga
Transcript
hello everyone the given question is state three characteristics that show chemical reaction has occurred to show three say that a chemical reaction has occurred so first one is evolution of gas evolution of gas also shows that a chemical reaction has occurred for example in many reaction evolution of gas takes place takes place for example when zinc reacts with sulphuric acid with forms zinc sulphate and evolve hydrogen gas
write the second one is formation of precipitate Prestige ITI so in many chemical reactions solid insoluble particles are formed these particles are known as precipitate right for example when Silver Nitrate Equus reacts with potassium chloride it forms a precipitate of silver chloride is of silver Chloride and potassium
nitrate right third one third characteristic change of state sometimes in chemical reaction occurs there is a change of state that means solid changes to liquid liquid changes to gas there is a if there is a change of state it is also characteristic of chemical reaction it shows that a chemical reaction has occurred for example when ammonia gaseous ammonia reacts with liquid HCL it warms ammonium chloride which is solid right this is the change of date I hope this question is clear
thank you
The Piece Of Calcium Rises To The Surface Covered With Hydrogen Bubbles
Within several tenths of a second the calcium dissolves, and from the suspension of hydroxide the water turns cloudy white. If you carry out the reaction in a test tube instead of a glass, you can observe the release of heat: the test tube quickly becomes hot. The reaction of calcium with water does not end with an effective explosion, but the two substances interact vigorously, making for an impressive sight. The experiment is safe.
If you take the bag with the remaining calcium out of the water and keep it in the air for a while, as a result of the continuing reaction it will heat up intensely, and the water remaining in the gauze will boil. If you filter part of the cloudy solution through a funnel into a glass, then if carbon dioxide is passed through the solution, a sediment will form. You do not need carbon dioxide gas for this, you can simply blow exhaled air into the solution through a glass straw.
Also Check: What Is The Definition Of Movement In Geography
Carbon And Its Function
The density of carbon keeps fluctuating like 2.25 g/cm³ or 1.30 ounces/in³ in graphite and it is 3.51 g/cm³ or 2.03 ounces/in³ in a diamond. Graphite’s melting point is 3500 or 6332. The boiling point in the extrapolated form is 4830 or 8726. Elemental carbon property is inert and thus, its insoluble in bases, water or diluted acids, and even organic solvents. However, at higher temperatures, it can bind with oxygen through which carbon monoxide and carbon dioxide are formed. When carbon reacts with oxidizing agents such as nitric acids, metallic acids, and potassium nitrate, C66 is formed. Considering the halogens, fluorine only makes the reaction to elemental carbon. Several metals bind with this element at different degrees of temperature to come up with carbides.
Video Review: Physical And Chemical Properties
2. physical chemical chemical physical physical
4. physical
6. The value of an extensive property depends upon the amount of matter being considered, whereas the value of an intensive property is the same regardless of the amount of matter being considered.
8. Being extensive properties, both mass and volume are directly proportional to the amount of substance under study. Dividing one extensive property by another will in effect cancel this dependence on amount, yielding a ratio that is independent of amount .
Don’t Miss: Michael Jackson Kids Real Father
Reactions Of Sodium With Organic Materials
Sodium has also found an application in organic chemistry it is used to obtain alcoholates from alcohols:
2CHOH + 2Na = 2CHONa + H ethanol, sodium ethylate CHONa forms).
Sodium is also used to extend carbohydrate chains in the Wurtz reaction:
CH-CH-Br + Br-CH-CH + 2Na = CH-CH-CH-CH + 2NaBr.
Sodium breaks bromine atoms off molecules of bromine ethyl. The connection of formed carbohydrate radicals takes place with formation of a longer molecule the two ethyl radicals CH-CH- connect, forming the n-butane molecule CH-CH-CH-CH).
Sodium is often used as a reducer in metallurgy and a drying agent of organic solvents in organic synthesis. The metal is also used for the manufacture of high-capacity batteries.
Origin Of Organic Compounds
There are various forms of production of organic substances depending on whether they are produced with the intervention or not of a living being:
- In-vivo processes . This term means inside living beings, and refers to those compounds and substances that living organisms synthesize to carry out their different processes of nutrition, reproduction , growth and regulation. Some of these compounds are:
- Proteins . They are macromolecules composed of amino acids at one end and a carboxyl group at the other end). They are very important to sustain the life of living beings.
- carbohydrates . They are molecules made up of carbon, hydrogen and oxygen. They are also called sugars and have a fundamental role in the life of plants and animals.
- lipids . They are composed mainly of carbon and hydrogen. They serve as energy reserves in living beings.
- nucleic acids . Its structure is made up of carbon, hydrogen, oxygen, nitrogen and phosphorus. They store the genetic information of living beings and transmit it from generation to generation.
Also Check: New England Colony Climate
Types Of Chemical Bond
There are three main types of chemical bond:
- Covalent bond . It occurs when non-metallic atoms of similar electronegativities come together to share electrons from their last orbit to form a stable chemical compound.
- Ionic bond . It consists of the union of metallic and non-metallic atoms, through a transfer of electrons from the metal to the non-metal . Thus, electrically charged ions are formed, cations and anions , which are electrostatically attracted to each other, constituting the bond and forming the different ionic chemical compounds. The atoms that form this type of bond have a large electronegativity difference.
- Metallic bond . It is the one that occurs between the metallic atoms of the same element in its solid state , which constitute extremely compact and narrow structures among themselves. It is a primary and strong bond, in which the atomic nuclei join each other surrounded by their electrons as in a cloud.
Definition Of Plasma The 4th State Of Matter
Plasma is considered to be the fourth state of matter. Here is how the term is used in science, especially chemistry and physics.
A different way to classify plasma is really as thermal or nonthermal. In thermal plasma, the electrons and heavier particles have been in thermal equilibrium or in the same temperature. In nonthermal plasma, the electrons are in a significantly greater temperature compared to ions and neutral particles .
The plasma ball toy is a typical example of plasma and how it behaves. Plasma is also found in neon lights, plasma displays, arc welding torches, and Tesla coils. Natural examples of plasma include lightning the aurora, the ionosphere, St. Elmos fire, and electrical sparks. While not often seen on Earth, plasma is the most abundant form of matter in the universe . The stars, interior of the Sun, solar wind, and solar corona consist of fully ionized plasma. The interstellar medium and intergalactic medium also contain plasma.
Read Also: Jonathan Thomas Child Of Rage Now
History Of Organic Chemistry
Organic chemistry became an important branch of chemistry in the 20th century , when new methods of investigating substances of plant and animal origin became possible.
However, as early as 1828 the German chemist Friedrich Wöhler had realized that an inorganic substance such as ammonium cyanate could be converted into an organic substance such as urea, present in the urine of many animals , thus contradicting the theory that organic compounds required the obligatory intervention of a living being.
Key Characteristics Of Us Chemistry Research

Chemists view the world at the atomic and molecular levels. They relate the properties of all substances to the detailed chemical compositions and atomic arrangements of all the chemical components. Understanding how the properties of substances are related to their molecular structures helps chemists design new molecules and materials that have the desired properties, allows them to develop or invent new types of transformations for carrying out the syntheses of these substances, and assists in designing ways to manufacture and process the new substances and materials.
A 2003 National Research Council report, Beyond the Molecular Frontier: Challenges for Chemistry and Chemical Engineering described some of the key structures and cultures of the disciplines and the common chemical bond that joins the two.1 Chemistry was described as an unusual natural science that pursues both discovery and creation. Chemists seek to discover the components of the chemical universefrom molecules to organized chemical systems such as materials, living cells, and whole organismsand to understand how these components interact and change over time. Synthetic chemists create new substances unknown in the natural world and develop novel transformations needed to make them. Chemical scientists produce tangible benefits to society when they design and engineer useful substances, such as new pharmaceuticals and polymeric materials.
Suggested Citation:
You May Like: What Is The Molecular Geometry Of Ccl4
V Chemical Properties Of Water
Water is a chemical as is any substance, despite the confusion and distrust of the public regarding the term chemical. Thus, water has lots of interesting chemical properties. It interacts intimately with components of food particularly as a solvent, due to its dipole moment and its tendency to form hydrogen bond. These interactions affect the chemical properties of nutrients, including their tendency to undergo oxidation or reduction, to act as acids or bases, and to ionize.
Physical Characteristics Of Gases
- Gases have a lower density and are highly compressible as compared to solids and liquids.
- They exert an equal amount of pressure in all directions.
- The space between gas particles is a lot, and they have high kinetic energy.
- The inter-molecular forces between these gas particles are negligible.
- These particles move at high speeds in all directions and hit each other, thus causing the gas to spread throughout the container they are kept in, evenly. This also causes them to exert pressure on the walls of the container.
- So, gases take the volume and shape of the container.
While a real gas has negligible inter-molecular forces of attraction, an ideal gas has zero inter-molecular forces of attraction because the molecules of an ideal gas move so fast, and they are so far away from each other that they do not interact at all. There is no ideal gas that exists naturally. However, gases behave most ideally at high temperatures and low pressure conditions. The behaviour of gases is governed by certain laws.
Recommended Reading: Geometry Workbook Answers Mcgraw Hill
Iv Macroscopic Properties Of Water
Collectively, water molecules exist as gas, liquid, or solid depending on the temperature and pressure. These phases of water exhibit collective or macroscopic properties such as phase transitions, crystal structures, liquid structures, vapor pressures, and volume-pressure relationships of vapor. In addition, energies or enthalpies for melting, vaporization and heating are also important for applications in food technology.
Thermodynamic constants for phase transitions given in Table 3 are those of pure water. Natural waters, of course, contain dissolved air, carbon dioxide, organic substances, microorganisms, and minerals. Water in food or used during food processing usually contains various organic and inorganic substances. These solutes modify the properties of water and caution should be taken to ensure proper values are applied in food technology.
The triple point and boiling points of water are defined as 273.16 and 373.16 K in the SI unit of temperature, respectively. Thus, the temperature differences can be in units of K or oC.
Physical And Chemical Properties
- Identify properties of and changes in matter as physical or chemical
- Identify properties of matter as extensive or intensive
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. We can observe some physical properties, such as density and color, without changing the physical state of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change. A physical change is a change in the state or properties of matter without any accompanying change in its chemical composition . We observe a physical change when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water . Other examples of physical changes include magnetizing and demagnetizing metals and grinding solids into powders . In each of these examples, there is a change in the physical state, form, or properties of the substance, but no change in its chemical composition.
Figure 1. Wax undergoes a physical change when solid wax is heated and forms liquid wax. Steam condensing inside a cooking pot is a physical change, as water vapor is changed into liquid water.
Read Also: What Is Vo In Physics
Chemical Property Vs Physical Property
You cant necessarily find out a substances chemical properties by looking at it. They are observed when that substance is undergoing a chemical change.
Water, for example, has a chemical structure of HO, or two hydrogen atoms bonded with one oxygen atom. Adding another oxygen atom gets you HO, hydrogen peroxide, which looks a lot like water both in its chemical formula and appearance in real life, but is a completely different substance.
We can drink water just fine, but you absolutely cannot drink straight hydrogen peroxide: it will chemically react with other substances in your body, damaging tissue and making you sick. That reaction is a very basic way of telling us about the important chemical property of toxicity.
Other chemical properties include a substances pH value and reactivity with water and oxygen. You have to test a substance by making it undergo a reaction to find out these properties. Physical properties such as color and density, on the other hand, can be observed without making the substance undergo a chemical change.
Characteristics Of A Chemical Bond
Polarity
Polarity is a characteristic of molecules formed by covalent bonds, and it depends directly on the nature of the bonded atoms .
If two atoms of the same element are bonded , there will be no difference between the electronegativity of each. In this way, the pair of electrons they share will be attracted to each of these atoms with the same force, so the distribution of electrical charges will be uniform in the molecule they form.
The same happens when atoms of different elements are bonded , but whose electronegativity difference is very small . Molecules formed in this way are called nonpolar or apolar and this type of bonding nonpolar covalent bond.
On the other hand, if two atoms of different elements are bonded , and the electronegativity difference between them is greater than 0.4 , then the most electronegative atom attracts towards itself with greater force the electrons of the bond, generating a non-uniform charge distribution in the structure. of the molecule they form. The molecules formed by this process are called polar and this type of bond is polar covalent bond.
Electrovalence
Ionic bonds are characterized by the fact that their atoms have a large electronegativity difference , to the extent that one loses and the other receives electrons when joining.
This electrical capacity in the atoms is known as electrovalence, since some elements are naturally more susceptible to being donors of electrons and others tend to be acceptors. .
Read Also: Mega Hack V5 Geometry Dash
What Is A Chemical Reaction
When two or more molecules combine to produce a new product, it is called a chemical reaction . Reactants are chemicals that combine to generate new compounds, whereas products are newly produced compounds.
Chemical reactions are important in a variety of businesses, cultures, and even our daily lives. They are always occurring in our environment, such as rusting of iron, pottery, and wine fermentation, to name a few. A chemical change must occur in a chemical reaction, which is commonly observed with physical changes such as precipitation, heat generation, colour change, and so on.
A reaction can occur between two atoms, ions, or molecules, in which they establish a new link without destroying or creating an atom, yet a new product is generated from reactants. The rate of reaction is influenced by variables such as pressure, temperature, and reactant concentration.