Effects On Lifestyle And Diet
In the late 19th Century and into the very early 20th Century, except for staple foods that needed no refrigeration, the available foods were affected heavily by the seasons and what could be grown locally. Refrigeration has removed these limitations. Refrigeration played a large part in the feasibility and then popularity of the modern supermarket. Fruits and vegetables out of season, or grown in distant locations, are now available at relatively low prices. Refrigerators have led to a huge increase in meat and dairy products as a portion of overall supermarket sales. As well as changing the goods purchased at the market, the ability to store these foods for extended periods of time has led to an increase in leisure time. Prior to the advent of the household refrigerator, people would have to shop on a daily basis for the supplies needed for their meals.
Earliest Forms Of Cooling
Before 1830, few Americans used ice to refrigerate foods due to a lack of ice-storehouses and iceboxes. As these two things became more widely available, individuals used axes and saws to harvest ice for their storehouses. This method proved to be difficult, dangerous, and certainly did not resemble anything that could be duplicated on a commercial scale.
Despite the difficulties of harvesting ice, Frederic Tudor thought that he could capitalize on this new commodity by harvesting ice in New England and shipping it to the Caribbean islands as well as the southern states. In the beginning, Tudor lost thousands of dollars, but eventually turned a profit as he constructed icehouses in Charleston, Virginia and in the Cuban port town of Havana. These icehouses as well as better insulated ships helped reduce ice wastage from 66% to 8%. This efficiency gain influenced Tudor to expand his ice market to other towns with icehouses such as New Orleans and Savannah. This ice market further expanded as harvesting ice became faster and cheaper after one of Tudor’s suppliers, Nathaniel Wyeth, invented a horse-drawn ice cutter in 1825. This invention as well as Tudor’s success inspired others to get involved in the ice trade and the ice industry grew.
How A Refrigerator Works
Here’s what’s happening inside your refrigerator as we speak! The left-hand side of the picture showswhat’s happening inside the chiller cabinet .The dotted line and pink area show the back wall and insulationseparating the inside from the outside.The right-hand side of the picture shows what’s going around the back of the fridge,out of sight.
You May Like: Who Is Paris Jackson’s Real Father
Why Does Cooling Take Time
Like everything else in our universe, refrigerators have to obey a fundamental law of physics called the conservation of energy. The gist is that you can’t createenergy out of nothing or make energy vanish into thin air: you can only ever convert energy into other forms.This has some very important implications for fridge users.
First, it busts the myth that you can cool your kitchen by leaving the refrigerator door open. Not true!As we’ve just seen, a refrigerator works by “sucking up” heat from the chiller cabinet with a cooling fluid,then pumping the fluid outside the cabinet, where it releases its heat. So if you remove a certain quantity of heat from inside your fridge, in theory, exactly the same amount reappears as heat around the back . Leave the door open and you’re simply moving heat energy from one part of your kitchen to the other.
Let’s try to put some rough figures to all this. The amount of energy it takes to change water’s temperature is called its specific heat capacity, and it’s 4200 joules per kilogram per degree celsius. It means you need to use 4200 joules of energy to heat or cool a kilogram of water by a single degree . So if you want to freeze a liter bottle of water from a room temperature of 20°C to a freezer-like 20°C, you’ll need 4200 × 1kg × 40°C, or 168,000 joules. If your refrigerator’s freezing compartment can remove heat at a power of 100 watts , that will take 1680 seconds or about half an hour.
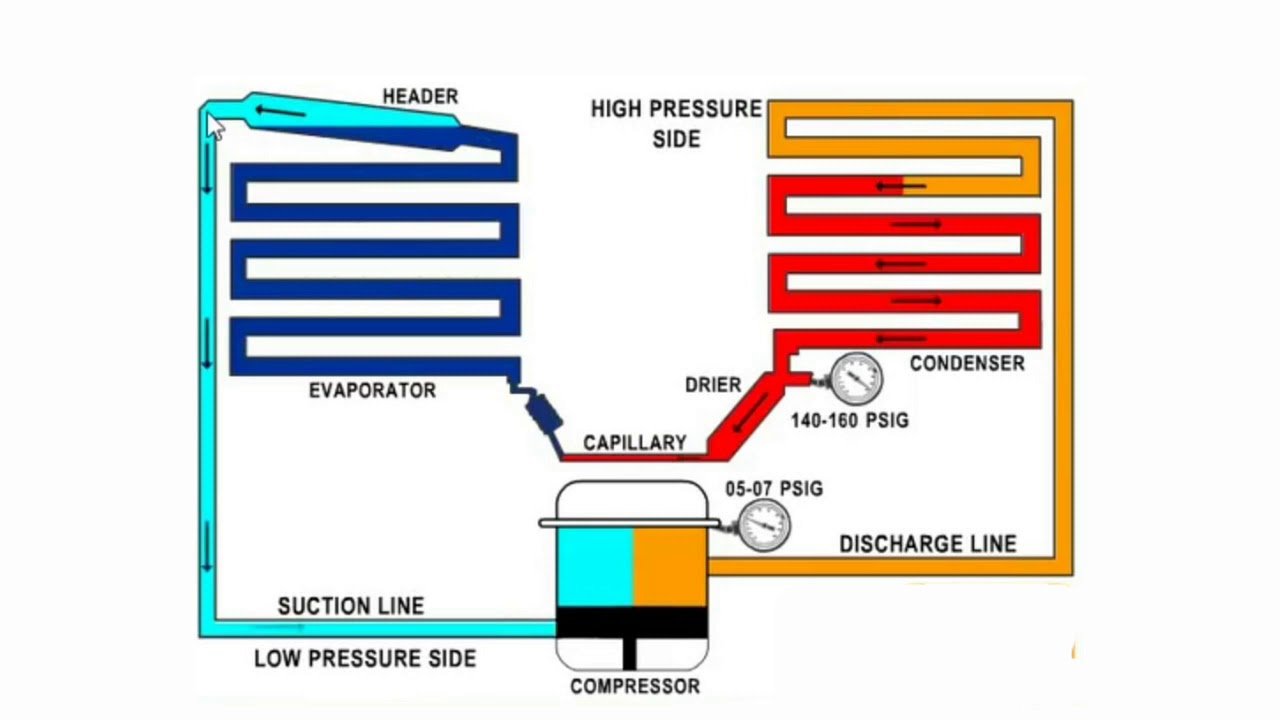
The Basic Vapor Compression Cycle And Components
Vapor compression refrigeration, as the name suggests, employs a compression process to raise the pressure of a working fluid vapor flowing from an evaporator at low pressure PL to a high pressure PH as shown in Figure 3. The refrigerant then flows through a condenser at the higher pressure PH, through a throttling device, and back to the low pressure, PL in the evaporator. The pressures PL and PH correspond to the refrigerant saturation temperatures, T1 and T5 respectively.
The T-S diagram for this real cycle, Figure 4, is somewhat different from the rectangular shape of the Carnot Cycle.
Figure 3. Basic vapor compression refrigerator.
Figure 4. T-S diagram for basic vapor compression cycle.
The cycle processes can be described as follows:
-
7-1 Evaporation of the liquefied refrigerant at constant temperature T1 = T7.
-
1-2 Superheating of the vapor from temperature T1 to T2 at constant pressure PL.
-
2-3 Compression from temperature T2 and pressure PL to temperature T3 and pressure PH.
-
3-4 Cooling of the super-heated vapor to the saturation temperature T4.
-
4-5 Condensation of the vapor at temperature T4 = T5 and pressure PH.
-
5-6 Subcooling of the liquid from T5 to T6 at pressure PH.
-
6-7 Expansion from pressure PH to pressure PL at constant enthalpy.
It is usual for the vapor-compression cycle to be plotted on a pressure-enthalpy diagram as shown in Figure 5.
The cycle calculations are described in detail in many textbooks .
Recommended Reading: How Did The Geography Of Greece Influence Greeks’ Interactions With Each Other
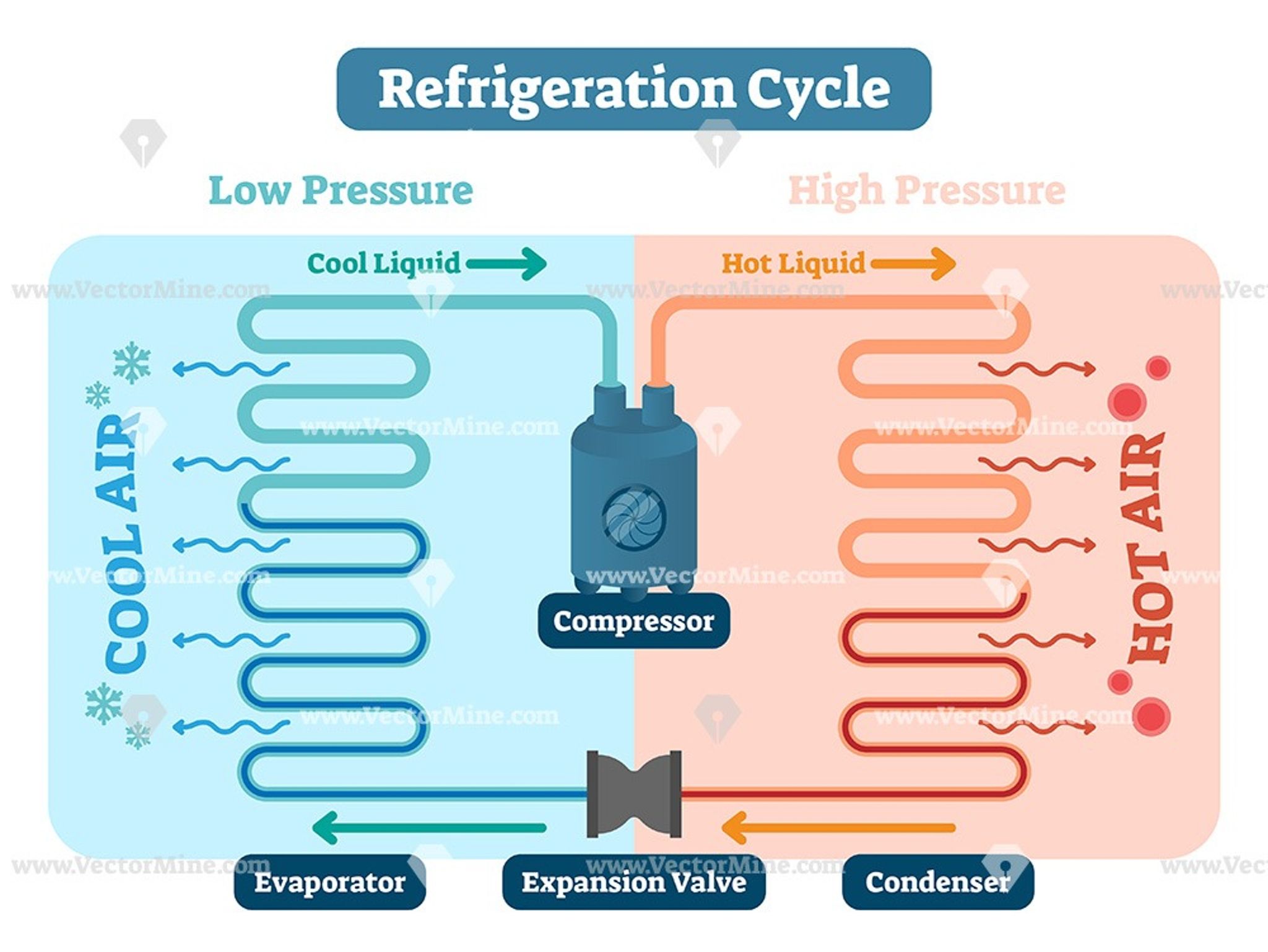
Refrigerator Function: How Does A Refrigerator Work
A refrigerator works in the following steps:
Now, lets discuss the working of a refrigerator in more detail.
The compressor, which is a critical component of the refrigerator, compresses the refrigerant gas. As it undergoes high pressure, the gas heats up. Now, this gas transports to the condenser coils located at the back of the fridge, where the coils help dissipate its heat so that it becomes cool enough to condense and convert back into its liquid phase.
Because the heat collected from the food items is given off to the surroundings via the condenser, it feels hot to the touch.
The high pressure liquid that we have now flows through the expansion valve. Think of the expansion valve as a small hole. On one side of the hole is high-pressure refrigerant liquid. On the other side of the hole is a low-pressure area .
Do you remember how a refrigerator works?
How To Move Heat With A Gas
Let’s step sideways a moment and look at how gases behave. If you’veever pumped up the tires on a bicycle,you’ll know that a bicycle pumpsoon gets quite warm. The reason is that gases heat up when you compress them. To make the tire supportthe weight of the bicycle and your body, you have to squeeze air intoit at a high pressure. Pumping makes the air a little bit hotter. Why? As yousqueeze the air, you have to work quite hard with the pump. The energy you use in pumping is converted into potential energy in the compressed gas: the gas in the tire is at a higherpressure and higher temperature than the cool air around you. If yousqueeze a gas into half the volume, the heat energy its moleculescontain fills only half as much space, so the temperature of the gasrises .
Artwork: Gases get hotter when you compress them into less volume, because you have to work topush their energetic molecules closer together. For example, when you inflate a bicycle tire, the pump sucks in air and squeezesit into less space. This forces its molecules together and makes it heat up.
Read Also: Holt Mcdougal Analytic Geometry Worksheets
Difference Between Heat Pump And Refrigerator
The difference between a heat pump and refrigerator is simple lets understand it:
A heat pump working principle is that a device transfers heat energy from a source of heat to the thermal reservoir. Heat pumps move thermal energy in the opposite direction of spontaneous heat transfer, by absorbing heat from a cold space and releasing it to a warmer one however, a refrigerator is a household appliance that is passed a cooler temperature than the external environment. It makes the objects cooler than the normal temperature.
Introduction To Basic Refrigeration
Before getting into the fundamentals of refrigeration, a few basic definitions should be considered:
A). Heat is a form of energy transferred by virtue of a difference in temperature. Heat exists everywhere to a greater or lesser degree. As a form of energy it can be neither created or destroyed, although other forms of energy may be converted into heat, and vice versa. It is important to remember that heat energy travels in only one direction from a warmer to a cooler object, substance, or area.
B). Cold is a relative term referring to the lack of heat in an object, substance, or area. Another definition describes it as the absence of heat, no process yet has been devised of achieving “absolute zero,” the state in which all heat has been removed from any object, substance, or area. Theoretically this zero point would be 459.69 degrees below zero on the Fahrenheit thermometer scale, or 273.16 degrees below zero on the Celsius thermometer scale.
C).Refrigeration, or cooling process, is the removal of unwanted heat from a selected object, substance, or space and its transfer to another object, substance, or space. Removal of heat lowers the temperature and may be accomplished by use of ice, snow, chilled water or mechanical refrigeration.
D). Mechanical refrigeration, is the utilization of mechanical components arranged in a “refrigeration system“ for the purpose of transferring heat.
F).Refrigeration system fundamental components.
Fig. 1-1: Simple Refrigeration System.
Recommended Reading: Movement In Geography Definition
Working Of The Refrigerator
Vapour compression refrigeration cycle is followed for the refrigeration process. In this process, the Evaporator, Compressor, Condenser and expansion valves are connected to tubes made of copper or steel.
Evaporator tube is placed throughout the refrigerator, when heat is absorbed the liquid refrigerant absorbs the heat and then converts into vapour. The heat absorbed is passed to the external environment through the compressor from vapour state to liquid state. This process repeats when the heat is absorbed and passed through the expansion valve to the evaporator. This process helps to keep the refrigerator cool always.
Read more about Murphys law.
| Related links |
The Heating And Cooling Cycle
By compressing gases into liquids, we can release heat by letting liquids expand into gases,we can soak up heat. How can we use this handy bit of physics to shiftheat from the inside of a refrigerator to the outside? Suppose we made a pipe that waspartly inside a refrigerator and partly outside it, and sealed so itwas a continuous loop. And suppose we filled the pipe with a carefullychosen chemical that easily changed back and forthbetween liquid and gas, which is known as the coolant or refrigerant.Inside the refrigerator, we could make the pipe suddenly get wider, sothe liquid coolant would expand into a gas and cool the chiller cabinetas it flowed through it. Outside the refrigerator, we could have something like a bicycle pump to compressthe gas, release its heat, and turn it back into a liquid. If the chemical flowed round and round theloop, expanding when it was inside the refrigerator and compressingwhen it was outside, it would constantly pick up heat from the insideand carry it to the outside like a heat conveyor belt. In this way, wecould constantly move heat from a cold place to a hotter one , which is not something that the laws of physics allow to happen automatically.
Photo: Humid air inside your fridge containswater vapor. When the refrigerator cools, this water turns to ice. Thecoldest part of your fridge is the icebox at the top. That’s becausethe expansion valve is placed right next to it.
Read Also: How Long Is The Ap Physics Exam
Types Of Domestic Refrigerators
Domestic refrigerators and freezers for food storage are made in a range of sizes. Among the smallest is a 4 L Peltier refrigerator advertised as being able to hold 6 cans of beer. A large domestic refrigerator stands as tall as a person and may be about 1 m wide with a capacity of 600 L. Some models for small households fit under kitchen work surfaces, usually about 86 cm high. Refrigerators may be combined with freezers, either stacked with refrigerator or freezer above, below, or side by side. A refrigerator without a frozen food storage compartment may have a small section just to make ice cubes. Freezers may have drawers to store food in, or they may have no divisions .
Refrigerators and freezers may be free-standing, or built into a kitchen.
Three distinct classes of refrigerator are common:
Example 1 The Best Cop: \\ Of A Heat Pump For Home Use
A heat pump used to warm a home must employ a cycle that produces a working fluid at temperatures greater than typical indoor temperature so that heat transfer to the inside can take place. Similarly, it must produce a working fluid at temperatures that are colder than the outdoor temperature so that heat transfer occurs from outside. Its hot and cold reservoir temperatures therefore cannot be too close, placing a limit on its COPhp. What is the best coefficient of performance possible for such a heat pump, if it has a hot reservoir temperature of 45.0ºC and a cold reservoir temperature of 15.0ºC?
Strategy
A Carnot engine reversed will give the best possible performance as a heat pump. As noted above, COP_}=\frac\\, so that we need to first calculate the Carnot efficiency to solve this problem.
Solution
Carnot efficiency in terms of absolute temperature is given by:
Eff_}=1-\frac}}}}\\.
The temperatures in kelvins are Th = 318 K and Tc = 258 K, so that
Eff_}=1-\frac}}=0.1887\\.
Thus, from the discussion above,
COP_}=\frac=\frac=5.30\\, or COP_}=\frac}}=\frac=5.30\\ so that Qh = 5.30 W.
Discussion
This result means that the heat transfer by the heat pump is 5.30 times as much as the work put into it. It would cost 5.30 times as much for the same heat transfer by an electric room heater as it does for that produced by this heat pump. This is not a violation of conservation of energy. Cold ambient air provides 4.3 J per 1 J of work from the electrical outlet.
Recommended Reading: Linear Algebra What Is Span
The Science Of Closed Loop Systems
In a closed loop system, nothing comes in, and nothing comes out the mixture inside the loops simply circulates up and down to transfer heat. Meanwhile, open loop systems require a constant supply of water.
Your fridge operates on a closed loop system because it is easy to maintain you never have to worry about refilling your mixture because it will never run out! Similarly, you never have to worry about refilling your Dandelion heat pumps ground loops with more water because everything stays contained within the closed loop system.
Not only is it much easier, but its also much safer to use closed loop systems. The refrigerant mixture inside your fridge never contaminates your food, and the water mixture inside the heat pumps ground loops never contaminates the groundwater below your home.
Closed loop systems also have an additional benefit. The Ideal Gas Law tells us that the pressure, volume, and temperature of gases are related, and since the volume of mixture inside a closed loop system never changes, then the temperature and pressure become indirectly proportionally related. In other words, this makes it easy to manipulate the mixture inside your fridges evaporator loops or inside your heat pumps ground loops: we simply compress the mixture to heat it up or expand it to cool it down! So, as the mixture within the loop heats up, it also absorbs heat from and cools its surroundings.
Heat Pump And Refrigerator: Applications
We have read about heat engines that convert heat energy into work and its application in various fields of thermodynamics. Can we think of a device that is the opposite of a heat engine, i.e. a device that helps in the conversion of work into heat? In this section, we will learn about the refrigerator and heat pump that works on the opposite principle of a heat engine.
Don’t Miss: Explain Why There Are Different Branches Of Chemistry
Working Principle Of Refrigerator
Refrigerators work on the second law of thermodynamics. In the process of refrigeration, unwanted heat is taken from one place and discharged into another. The common refrigerator which we have in our homes, works on the principle of evaporation. A refrigerant is a substance used in a heat cycle to transfer heat from one area, and remove it to another. A refrigerant when passed through the food kept in the refrigerator, it absorbs heat from these items and transfers the absorbed heat to the surrounding with less temperature.