Applications Of Quantitative Analysis In The Business Sector
Business owners are often forced to make decisions under conditions of uncertainty. Luckily, quantitative techniques enable them to make the best estimates and thus minimize the risks associated with a particular decision. Ideally, quantitative models provide company owners with a better understanding of information to enable them to make the best possible decisions.
Project Management
One area where quantitative analysis is considered an indispensable tool is in project management. As mentioned earlier, quantitative methods are used to find the best ways of allocating resources, especially if these resources are scarce. Projects are then scheduled based on the availability of certain resources.
Production Planning
Quantitative analysis also helps individuals to make informed product-planning decisions. Lets say a company finds it challenging to estimate the size and location of a new production facility. Quantitative analysis can be employed to assess different proposals for costs, timing, and location. With effective product planning and scheduling, companies will be more able to meet their customers needs while maximizing their profits.
Finance
Purchase and Inventory
More On Quantitative Analysis Relying On A Chemical Reaction
Gravimetric analysis: Here the said substance is precipitated into an insoluble form which is filtered, dried and weight is measured as a function of quantity.
Ex: Barium sulfate + Sodium carbonate Barium carbonate + Sodium sulfate.
Here Barium sulfate is soluble and is converted to Barium carbonate an insoluble form by the addition of sodium carbonate.
The insoluble precipitate is weighed for the quantity of Barium ion in the given sample.
Titrimetric analysis:Titration is a method wherein the volume of reagent required to complete the reaction with the substance of interest is noted using reactions like acid-base titration, oxidation-reduction, complex-forming or precipitation reactions.
Ex: HCl + NaOH NaCl + H2O. Once the reaction reaches completion, the endpoint is indicated by a change in color of an indicator added to the reaction mixture.
Why Quantitative Analysis Is Important
It’s important to know the quantity of all or part of a sample for several reasons.
If you’re performing a chemical reaction, quantitative analysis helps you predict how much product to expect and to determine your actual yield.
Some reactions take place when the concentration of one component reaches a critical level. For example, an analysis of radioactive material might indicate there is enough of a key component for the specimen to undergo spontaneous fission!
Quantitative analysis is crucial to the formulation and testing of food and drugs, as it is used to measure nutrient levels and provide an accurate accounting of dosage.
It is also critical in determining the level of contaminants or the impurity of a sample. While qualitative analysis might be able to determine the presence of lead in the paint on a toy, for example, quantitative analysis detects how much concentration exists.
Medical tests rely on quantitative analysis for information about a patient’s health. For example, quantitative analysis techniques can determine blood cholesterol levels or the ratio of lipoproteins in plasma or the amount of protein excreted in urine. Here again, quantitative analysis complements qualitative analysis, since the latter identifies the nature of a chemical while the former tells you how much there is.
Quantitative tests of a mineral may be used to determine whether it’s practical to mine it for a specific element or compound.
Also Check: Eoc Fsa Practice Test Algebra 2 No Calculator Portion Answers
Importance Of Analytical Chemistry
Analytical chemistry is the branch which is taught in almost all schools and colleges. But the applications of it are made in pharmaceutical industries, food factories, chemical industries, agricultural industries and in scientific laboratories. The tools used for this purpose are quite expensive which one cannot afford at home.
Estimation Of Carbon And Hydrogen
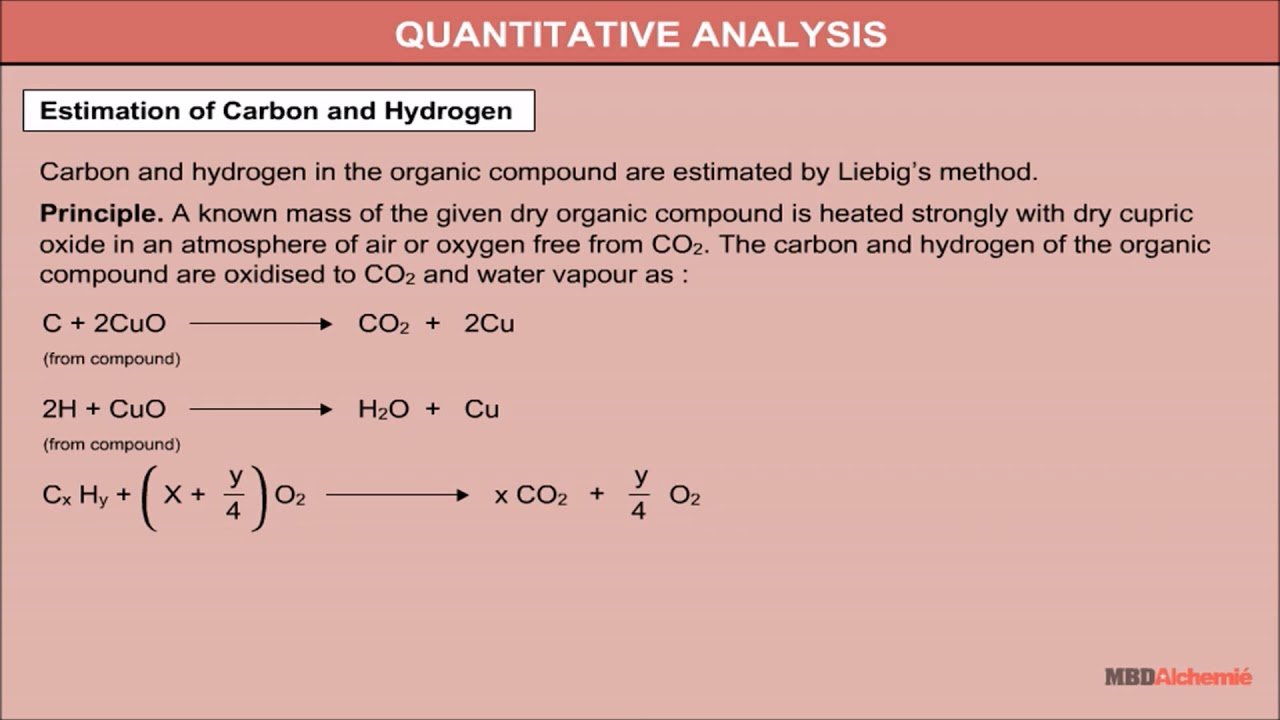
These two elements are always estimated together by Liebigs combustion method. A weighted amount of the compound is heated strongly with excess copper oxide in an atmosphere of air or oxygen. The constituents hydrogen and carbon are thus oxidised to water and carbon dioxide, which are collected separately and weighed. The percentage of carbon and hydrogen in the compound can be calculated as given below.
% C = 12/44 x Mass of CO2 formed/ Mass of the substance x 100
% H = 1/18 x Mass of H2O formed / Mass of the substance x 100
Also Check: Paris Jackson Biological Parents
Classical Methods Instrumental Methods
Quantitative analysis is a chemical analysis performed to find the amount of each component present in a material. It is done by either a classical or instrumental procedure.
A quantitative investigation means that the amount or relative amount of each component present is determined. In a pure substance, the entire mass, or 100%, is composed of a single component. In materials composed of two or more substances, a quantitative investigation would determine the mass or relative mass present for each component within the sample. It is not always necessary to find quantitative values for all components that make up a substance. In most cases it is sufficient to analyze the material for one or perhaps more components of interest. The amount of active medicine within an antacid tablet, for example, is significant, whereas the fillers, binders, colorants, and flavoring agents present are of lesser importance.
A quantitative analysis involves more than simply measuring the amount of a component present in a sample
The sample must first be prepared for measurement, usually by placing it in solution if it is not already in soluble form. With complex substances a preliminary separation of the desired component is often necessary to prevent other substances present from interfering with the selected analytical method.
TABLE 1. INSTRUMENTAL TECHNIQUES
S Used In Quantitative Analysis
Several methods are used to quantify a sample. These may be broadly classified as either physical or chemical methods.
Physical methods measure a physical property, such as adsorption of light, density, and magnetic susceptibility. Examples of physical methods include:
- Fourier Transform Infrared Spectroscopy
- Atomic Emission Spectroscopy
- Combustion analysis
- Inert gas fusion
Often physical and chemical methods overlap. In addition, mathematics is used in quantitative analysis. Statistics are particularly useful for analyzing data.
The primary tool for quantitative analysis is the analytical balance or scale, which is used to measure mass precisely. Glassware, such as the volumetric flask, is also important. For analytical chemistry, a typical balance measures mass to 0.1 of a milligram. A sensitivity of about a thousand times is needed for microanalytical work.
Don’t Miss: Chapter 7 Test Algebra 1 Answer Key
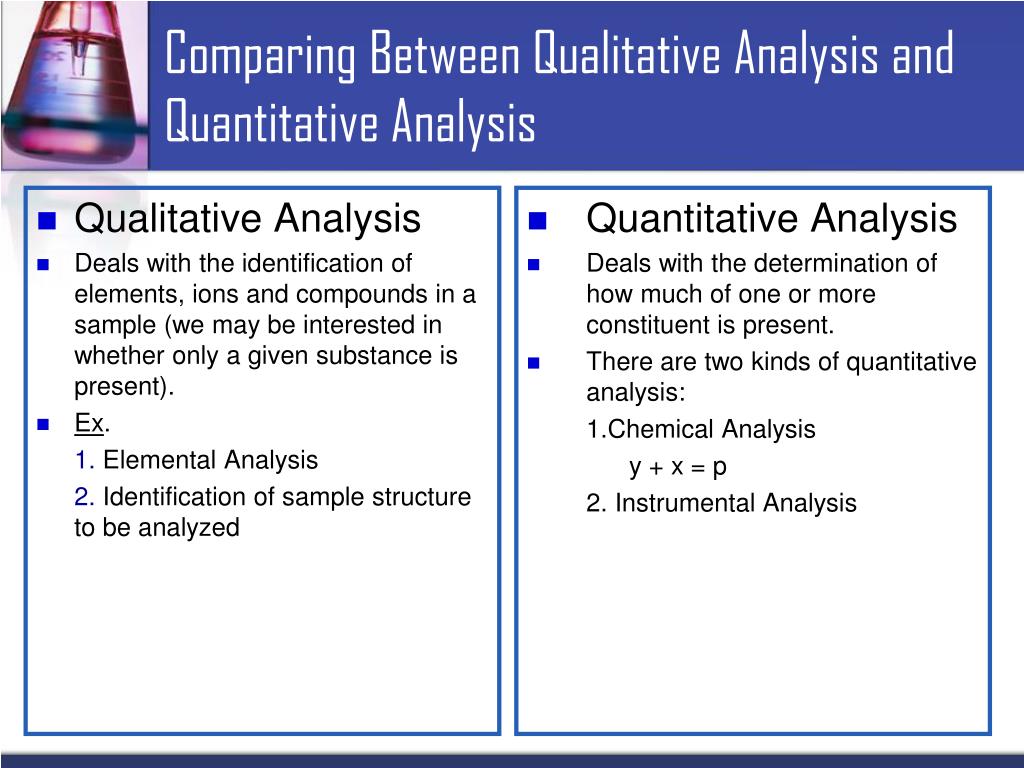
Qualitative And Quantitative Analysis
In this chapter, we will discuss the Qualitative and Quantitative Analysis of Various Elements in an Organic Compound. In Qualitative Analysis we try to identify which element is present and in the quantitative analysis, we try to calculate the % amount of that particular element present in an organic compound. Organic Compounds by default are made up of Carbon and Hydrogen. This chapter is crucial for both Boards and competitive exams.
What Are Qualitative Tests In Chemistry
5/5Qualitativequalitativequalitative testschemicaltests
Herein, what are qualitative tests?
qualitative testing. The process of determining whether or not a particular chemical is present in a sample. Some types of business specialize in the service of performing qualitative testing of samples provided by customers who wish to know what is in them.
Likewise, what is qualitative chemistry? In chemistry, qualitative analysis is the determination of the chemical composition of a sample. Qualitative analysis can tell you whether an atom, ion, functional group, or compound is present or absent in a sample, but it doesn’t provide information about its quantity.
Accordingly, what is a quantitative test in chemistry?
In analytical chemistry, quantitative analysis is the determination of the absolute or relative abundance of one, several or all particular substance present in a sample.
What is qualitative and quantitative analysis in chemistry?
Qualitative analysis tells ‘what’ is in a sample, while quantitative analysis is used to tell ‘how much’ is in a sample. The two types of analysis are often used together and are considered examples of analytical chemistry.
You May Like: Uber Hacker Geometry Dash
Qualitative And Quantitative Analysis In Biochemistry
A qualitative study allows us to make inferences about the biochemical pathway, such as the impact of specific processes, metabolites, or pathway segments on the overall system.
A quantitative study allows us to understand how much compound is present in the biomolecules. People with diabetes can use quantitative whole blood glucose measurements to track their blood glucose levels. The quantitative method is useful in the analysis of urea, protein, carbohydrate, etc.
What Is Quantitative Measurement In Chemistry
Quantitative means calculating a quantity-setting it to a value. For instance, you might calculate the rate of a reaction by seeing how many seconds it takes for a change to occur, like a piece of magnesium ribbon to dissolve in acids of different concentrations. Qualitative means, without a meaning being calculated.
Recommended Reading: Structural Formula Of Ccl4
What Does Analytical Chemistry Have To Do With Everyday Life
Analytical chemistry has an important role in everyday life. It helps to measure the simple medical tests like serum cholesterol, urine ketones, and blood glucose level, Analytical techniques also help in determining the levels of toxic waste in the body like uric acid, cholesterol, drugs and some salts. Soil testing, water testing are also important roles of Analytical chemistry in everyday life.
Optical Methods Of Quantitative Analysis
Absorption methods like visible spectroscopy i.e colorimetry, ultraviolet spectrophotometry, infrared spectrophotometry, etc.
Here the substance in the sample absorbs a certain wavelength of light and this absorption is determined as a function of quantity.
Visible spectroscopy and UV spectroscopy rely on the light-absorbing property of the substance.
Every substance absorbs light to a maximum extent at a specific wavelength. This wavelength of light is called lambda max.
Infrared spectroscopy is a bit different than the above. It is used to check the bonds between atoms and molecules.
Emission methods involve techniques like emission spectroscopy, fluorimetry, etc.. wherein the characteristic light emitted by a substance is recorded as a function of quantitative analysis.
Fluorimetryis based on the ability of a sample to absorb and re-emit light of a certain wavelength.
When light is passed on to the sample at a specific wavelength, the electrons in the atoms get into exited state.
They come back to the ground state by emitting light of a certain wavelength as fluorescence.
This fluorescence emitted is measured to estimate the quantity of the sample.
You May Like: Elastic Force Formula
What Is The Difference Between Quantitative And Qualitative Chemistry
The difference between qualitative and quantitative analysis in chemistry is that the qualitative analysis does not measure the amount of the substance but measures the quality of that material whereas quantitative analysis in chemistry gives the absolute or relative quantity regarding the concentration of one or more substances present in a sample or compound.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Qualitative Analytical Methods And Selectivity And Sensitivity
The complexity of the qualitative analytical method varies depending on the nature of the sample to be analyzed. There are two special characteristics in a qualitative analytical method. It should be a specific one and sensitive one. Specificity involves the ability to detect a certain component or element in the presence of the other components. Sensitivity involves the ability to detect the testing element, even if it is present in trace quantities. In other words, sensitivity is defined as the smallest quantity of an element / compound that can be detected by a given method. Some methods are very sensitive and for some, it needs to have a fairly high concentration for the detection.
Example: Identification of SO42- ions
Method 1: Using mercury nitrate solution
When mercury nitrate is added to a solution containing sulphate ions, a yellow precipitate of basic mercury sulphate is formed. This is a very sensitive test, which means it gives a precipitate even if the SO42- concentration is very low.
Method 2: Using silver nitrate solution
When silver chloride is added to a sulphate solution, a crystalline precipitate of silver sulphate is formed. This occurs only in concentrated solutions .
Read Also: Calculating Half-life
Qualitative Vs Quantitative Analysis In Chemistry
Qualitative analysis in chemistry is a branch of chemistry that analyses the chemical composition of a sample. Quantitative analysis in chemistry is a branch of chemistry that deals with the quantities of different components in a sample. Details Qualitative analysis in chemistry gives the presence or absence of different chemical components in a sample. Quantitative analysis in chemistry gives the amount of different chemical components present in a given sample. Techniques Qualitative analysis in chemistry uses techniques such as distillation, extraction, and change in colour, chromatography, etc. Quantitative analysis in chemistry uses techniques such as titrations, gravimetric analysis, combustion analysis, AES, etc.
Branches Of Qualitative Analysis
The two main branches of qualitative analysis are organic qualitative analysis and inorganic qualitative analysis .
Inorganic analysis looks at the elemental and ionic composition of a sample, usually by examination of ions in aqueous solution. Organic analysis tends to look at types of molecules, functional groups, and chemical bonds.
Read Also: What Does Biomass Mean In Biology
Qualitative And Quantitative Analysis Chemistry
Qualitative and quantitative analysis is analytical techniques in Chemistry that are used for giving details about the components in an unknown sample.
Qualitative analysis in Chemistry gives details of the presence or nonappearance of different chemical components in an unknown sample, while quantitative analysis gives the measure of various chemical components present in a given sample.
Most often, both the techniques are used together, i.e., the use of qualitative analysis followed by quantitative analysis.
On this page, we will focus on qualitative chemical analysis, qualitative inorganic analysis, and understand what qualitative analysis is in chemistry in detail.
Detection Of Nitrogen Sulphur And Halogens
Lassaignes Test: Small pea-sized sodium is heated gently in a fusion tube until it forms a shining globule. A small amount of the sample is added to it and the fusion tube is reheated to red hot. It is then, plunged in a mortar with 10-15 mL water and nicely grinded. Filtered hot. The filtrate is known as sodium extract or lassaignes extract.
| Element | ||
| Na+C+N+SNaCNS | As in test for nitrogen instead of green or blue colour, blood red coloration confirms presence of N and S both. | NaCNS + FeCl3 Cl2 + NaCl |
Read Also: Chapter 7 Cumulative Review Answers
Different Methods Of Quantitative Analysis
The study of the absolute and relative abundance in a given sample, for the determination of the specific properties of certain substances present in the sample. For best use of quantitative analysis chemistry and chemical, methods have been developed so much that there are numerous methods and techniques to identify and characterize any sample quantitatively. Although there are numerous methods and techniques developed, knowing a sample or the composition of the sample is of significant importance to eliminate any possible tests for further characterisation. Two of the common classifications under which various methods of analysis which students can also find in any textbook of quantitative chemical analysis. They are classified as under:
The benefit of quantitative chemical analysis is that they are very generalised in nature. They can be applied to understand a wide range of analytes especially while performing quantitative inorganic analysis. Examples of some of these general characteristics of the methods are stated below:
Other examples of methods that are part of the quantitative chemical analysis are the Leibig method or Dumas method or Kjeldahls method and the Carius method for the estimation of the organic compounds. These quantitative analysis methods can also be applied for the mass spectrometry on the biological samples that can be determined by the relative abundance of the ratio of the specific proteins, and indications of diseases like cancer.
Key Difference Qualitative Vs Quantitative Analysis In Chemistry
Qualitative and quantitative analysis in chemistry are the major types of analytical techniques used in chemistry to determine the chemical composition of sample qualitatively and quantitatively. The key difference between qualitative and quantitative analysis in chemistry is that qualitative analysis in chemistry gives the presence or absence of different chemical components in a sample whereas quantitative analysis in chemistry gives the amount of different chemical components present in a given sample.
Read Also: Beth Thomas Married
Applications Of Analytical Chemistry
Some important applications of this branch of chemistry are listed below.
- The shelf lives of many medicines are determined with the help of analytical chemistry.
- It is used to check for the presence of adulterants in drugs.
- Soil can be tested to check for appropriate concentrations of minerals and nutrients that are necessary for plant growth.
- It is employed in the process of chromatography where the blood samples of a person are classified.
- The concentration of the pesticide residues and the contaminants in a given food sample can also be determined via analytical chemistry.
- It also has many important applications in medicine, with its use in the testing of cholesterol and glucose levels in a blood sample.
- Analytical chemistry is an integral part of forensic science, clinical analysis, and even environmental analysis.
Thus, a brief introduction to the field of analytical chemistry is provided in this article. To learn more about this branch of chemistry along with other branches, such as inorganic chemistry, register with BYJUS and download the mobile application on your smartphone.
What Is Qualitative Analysis In Chemistry
Qualitative analysis in Chemistry is if two types:
-
Qualitative organic analysis
Qualitative organic analysis determines the chemical bonds and functional groups in a sample.
2. Qualitative Inorganic Analysis
Qualitative inorganic analysis frequently determines the ions in a given sample.
What did we understand so far?
So, in a nutshell, qualitative analysis chemistry uses techniques, like distillation, extraction, and change in colour, chromatography, etc, to determine the composition of a sample.
In other words, these techniques are helpful in determining the presence of different chemical components in a sample.
Now, lets focus on examples of qualitative analysis in chemistry:
Don’t Miss: Angle Addition Postulate Homework 4