Other Forms Of Energy
While we are most likely to encounter these six forms of energy in daily life, they are not the only ways that energy can be seen. In general, however, other forms of energy are really special descriptions of the six forms we’ve discussed. Sound energy, for example, can create concussive forces through vibration of air particles. This is really a specific form of mechanical energy. The key point is that you will encounter energy in a large variety of ways.
Comprehension Checkpoint
__________ energy has to do with the motion of molecules, while __________ energy has to do with the flow of electrons.
Example For Transformation Of Heat Into Light
A hot body glows and emits light. For example, by the Nuclear fusion process, a huge amount of heat produces in the Sun and therefore the Sun glows and emits light. Similar thing happens in the electric bulbs too. The electrical energy heats the filament of a bulb and the bulb glows and emits light.
Principle Of Conservation Of Energy
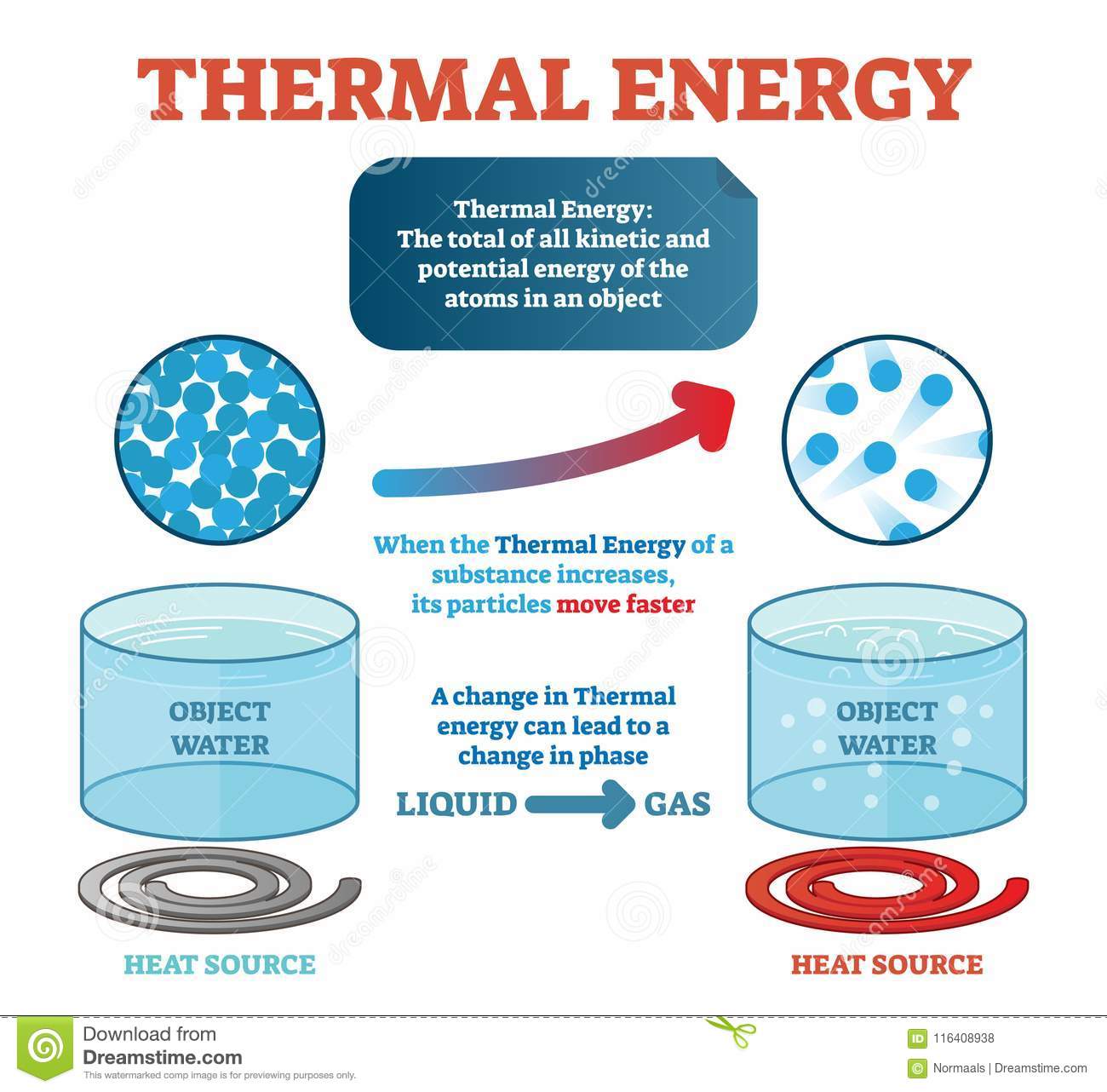
One of the most wonderful properties of the Universe is that energy can be transformed from one type to another and transferred from one object to another. Moreover, when transformed from one type to another and transferred from one object to another, the total amount of energy is always the same. It is one of the elementary properties of the Universe.
In thermodynamics, the concept of energy is broadened to account for other observed changes. The principle of conservation of energy is extended to include a wide variety of ways systems interact with their surroundings. The only ways the energy of a closed system can be changed are through the transfer of energy or . Further, based on the experiments of Joule and others, a fundamental aspect of the energy concept is that energy is conserved. This principle is known as the first law of thermodynamics. The first law of thermodynamics can be written in various forms:
In words:
Equation form:
Eint = Q W
where Eint represents the internal energy of the material, which depends only on the materials state , Q is the net heat added to the system, and W is the net work done by the system. We must be careful and consistent in following the sign conventions for Q and W. Because W in the equation is the work done by the system, then if work is done on the system, W will be negative, and Eint will increase.
Differential form:
dEint = dQ dW
Also Check: How To Study Ap Human Geography
Observation Of Quantum Objects
The act of observation is a topic of considerable discussion in quantum physics. Early in the field, scientists were baffled to find that simply observing an experiment influenced the outcome. For example, an electron acted like a wave when not observed, but the act of observing it caused the wave to collapse and the electron to behave instead like a particle. Scientists now appreciate that the term “observation” is misleading in this context, suggesting that consciousness is involved. Instead, “measurement” better describes the effect, in which a change in outcome may be caused by the interaction between the quantum phenomenon and the external environment, including the device used to measure the phenomenon. Even this connection has caveats, though, and a full understanding of the relationship between measurement and outcome is still needed.
Examples Of Energy Of 1 Joule
One joule in everyday life and in science corresponds to approximately:
- The kinetic energy of an object with mass 1 kg moving at 2 1.4 m/s.
- The kinetic energy of a 50 kg object moving very slowly approximately 0.72 km/h.
- The energy required to lift a medium-size apple 1 meter vertically from the surface of the Earth.
- The heat required to raise the temperature of 1 g of water by 0.24 °C.
- The heat required to evaporate of 0.00044 g of liquid water at 100°C.
- The amount of electricity required to light a 1 watt LED for 1 s.
- Is released by approximately 3.11010 fissions in a nuclear reactor.
You May Like: What Is Invasive Species In Biology
Example Of Energies In Electronvolts
- Thermal neutrons are neutrons in thermal equilibrium with a surrounding medium of temperature 290K . Most probable energy at 17°C for Maxwellian distribution is 0.025 eV .
- Thermal energy of a molecule is at room temperature about 0.04 eV.
- Approximately 1 eV corresponds to an infrared of wavelength 1240 nm.
- Visible light photons have energies in range 1.65 eV 3.26 eV .
- The first resonance in n + 238U reaction is at 6.67 eV , which corresponds to the first virtual level in 239U, has a total width of only 0.027 eV, and the mean life of this state is 2.4×10-14s.
- Ionization energy of atomic hydrogen is 13.6 eV.
- Carbon-14 decays into nitrogen-14 through beta decay . The emitted beta particles have a maximum energy of 156 keV, while their weighted mean energy is 49 keV.
- High energy diagnostic medical x-ray photons have kinetic energies of about 200 keV.
- Thallium 208, which is one of nuclides in the 232U decay chain, emits gamma rays of 2.6 MeV which are very energetic and highly penetrating.
- Typical kinetic energy of alpha particle from radioactive decay is about 5 MeV. It is caused by the mechanism of their production.
- The total energy released in a reactor is about 210 MeV per 235U fission, distributed as shown in the table. In a reactor, the average recoverable energy per fission is about 200 MeV, being the total energy minus the energy of the energy of antineutrinos that are radiated away.
- Cosmic ray can have energies of 1 MeV 1000 TeV.
Energy Conversion: Transfer And Transform
We know the energy can be transferred from one form to another, the movement of energy from one location to another is known as energy transfer. We notice various energy transformations happening around us.
Following are the four ways through which energy can be transferred:
- Mechanically By the action of force
- Electrically Electrically
The process which results in the energy changing from one form to another is known as energy transformation. While energy can be transformed or transferred, the total amount of energy does not change this is called energy conservation.
Read More: Energy Conversion
Don’t Miss: What Is Insulator In Physics
Energy In The Human Body
Remember back at the beginning of this article where the phrases, I just dont have the energy today, and, Those kids need to burn off some energy were mentioned? Humans make use of energy all the time, and not just from their electronic devices. Both the large motions of your body and small processes within your body require energy.
It takes energy to run, hike, swim or even just brush your teeth. Remember kinetic energy? When you move, you are doing so via kinetic energy. That energy has to come from somewhere.
Many invisible processes that go on in your body also require energy, such as breathing, circulating your blood, digesting and so on.
Where do humans get their energy from? Food, of course! The food you eat has stored chemical energy within it. When that food makes its way into your stomach, your stomach acid breaks down the food, and certain molecules from the food make their way to all of the different places in your body that might need energy. Then, when the need arises, energy is obtained via a small chemical reaction.
Now, if you dont eat all day and do a lot of running around, you expend a lot of energy and will feel drained until you eat and provide your body with more of what it needs.
Related Articles
Conversion Of Chemical Energy Into Electricity
Earlier we became to know that a battery stores energy in the form of chemical energy which comes from the electrical energy while its charging. The reverse case also exists. While discharging of a battery, the stored chemical energy supplies the electric energy to the connected devices like mobiles, laptops, clocks, etc.
Read Also: What Jobs Require Biology Degree
Conversion Of Electrical Energy Into Heat Energy
We got electrical energy from the Sun and now will use it to transform into other forms. The conversion of electrical energy into heat is another example of the transformation of energy. This type of energy transformation is used in induction heaters for cooking and heating water. Electrical inductions convert electrical energy into heat and we can cook by using the heat generated.
The Importance Of Energy To Science
From astronomy to zoology, all forms of natural science rely on an understanding of energy to some degree. In the physical sciences, our understanding of energy flow helps to predict chemical reactions, determine the trajectory of objects, and many other processes. In the life sciences, energy is used to study how enzymes work and why different biomolecules interact in certain ways. Energy is a fundamental concept for all students of science, and it is a cornerstone for existence at large.
Recommended Reading: What Does Range In Math
Secondary Energy Sources Energy Carriers
Secondary energy sources, also called energy carriers, are derived from the transformation of primary energy sources. They are called energy carriers because they move energy in a useable form from one place to another. The well-known energy carriers are:
Electricity and hydrogen are made from primary energy sources such as coal, natural gas, nuclear energy, petroleum, and renewable energy sources. Electricity is particularly useful since it has low entropy and can be converted into other forms of energy very efficiently. Simply, we cannot say that hydrogen has the potential to offset fossil fuels.
Secondary energy sources are used because their use is easier than using a primary energy source. For example, using electricity for lighting is safer than using petroleum in candles or kerosene lamps.
On the other hand, any conversion of primary energy to energy carrier is associated with some inefficiency. Therefore when dealing with the secondary energy source, we always have to consider the way, how the carrier was made.
Misuse Of Watts Per Hour
Many compound units for various kinds of rates explicitly mention units of time to indicate a change over time. For example: miles per hour, kilometres per hour, dollars per hour. Power units, such as kW, already measure the rate of energy per unit time . Kilowatt-hours are a product of power and time, not a rate of change of power with time.
Watts per hour is a unit of a change of power per hour, i.e. an acceleration in the delivery of energy. It is used to measure the daily variation of demand , or ramp-up behavior of . For example, a power plant that reaches a power output of 1 MW from 0 MW in 15 minutes has a ramp-up rate of 4 MW/h.
Other uses of terms such as watts per hour are likely to be errors.
Don’t Miss: What Is The Definition Of Work In Physics
The Law Of Conservation Of Energy
A fundamental fact of nature is that energy can neither be created nor destroyed. This is summarized in the law of conservation of energy. This law states that the total energy of an isolated system remains constant.
While the total energy remains constant, it can and often does change form. Potential might change into kinetic, kinetic might change into thermal energy and so on. But the total amount always remains the same.
Its important to note that this law specifies an isolated system. An isolated system is one in which can in no way interact with its surroundings. The only possibly perfectly isolated system in the universe is, well, the universe itself. However, its possible to make many systems on Earth that are close to being isolated
Energy conversion can happen in many ways, usually from stored energy being released as kinetic energy of some sort or as radiant energy.
Chemical energy, for example, can be released during chemical reactions. During such a reaction it changes from chemical potential energy into some other form, which might include radiant energy or heat energy.
Nuclear energy is released during a nuclear reaction. This is where Einstein’s famous E = mc2 equation comes into play . The mass of a nucleus that splits apart to release energy will be slightly lighter in the end by an amount determined by Einsteins formula. As crazy as it sounds, mass itself can be considered a form of potential energy.
Work Energy And Power
Work, Energy and Power are fundamental concepts of Physics. Work is said to be done when a force applied to an object causes a displacement of the object. We define the capacity to do the work as energy. Power is the work done per unit of time. This article discusses work, energy and power in detail.
Read Also: What Is Hoh In Chemistry
So What Is Energy Then
The reason it is so hard to define is because its an abstract notion. In physics, the concept of energy is really just a kind of shorthand, a tool to help balance the books. It is always conserved so is incredibly useful in working out the results of any kind of physical or chemical process.
There is no physical essence of energy, and no such thing as pure energy. It is always carried by something, usually in the form of movement.
The classic example of kinetic energy is a billiard ball rolling across a table. The heavier the ball, the faster it moves, the more energy it carries. In other words, the more painful it will be if it pops off the table and lands on your little toe.
Another form of kinetic energy is known as heat. The temperature of something is a direct measurement of how fast the atoms inside it are moving. In a hot cup of coffee, the water molecules are racing around at a fast clip, slowing down as the cup cools.
Throw an iron bar into the fire and its atoms start moving faster too, although in this case the atoms are bound in position, and so the movement is the form of a jiggling vibration.
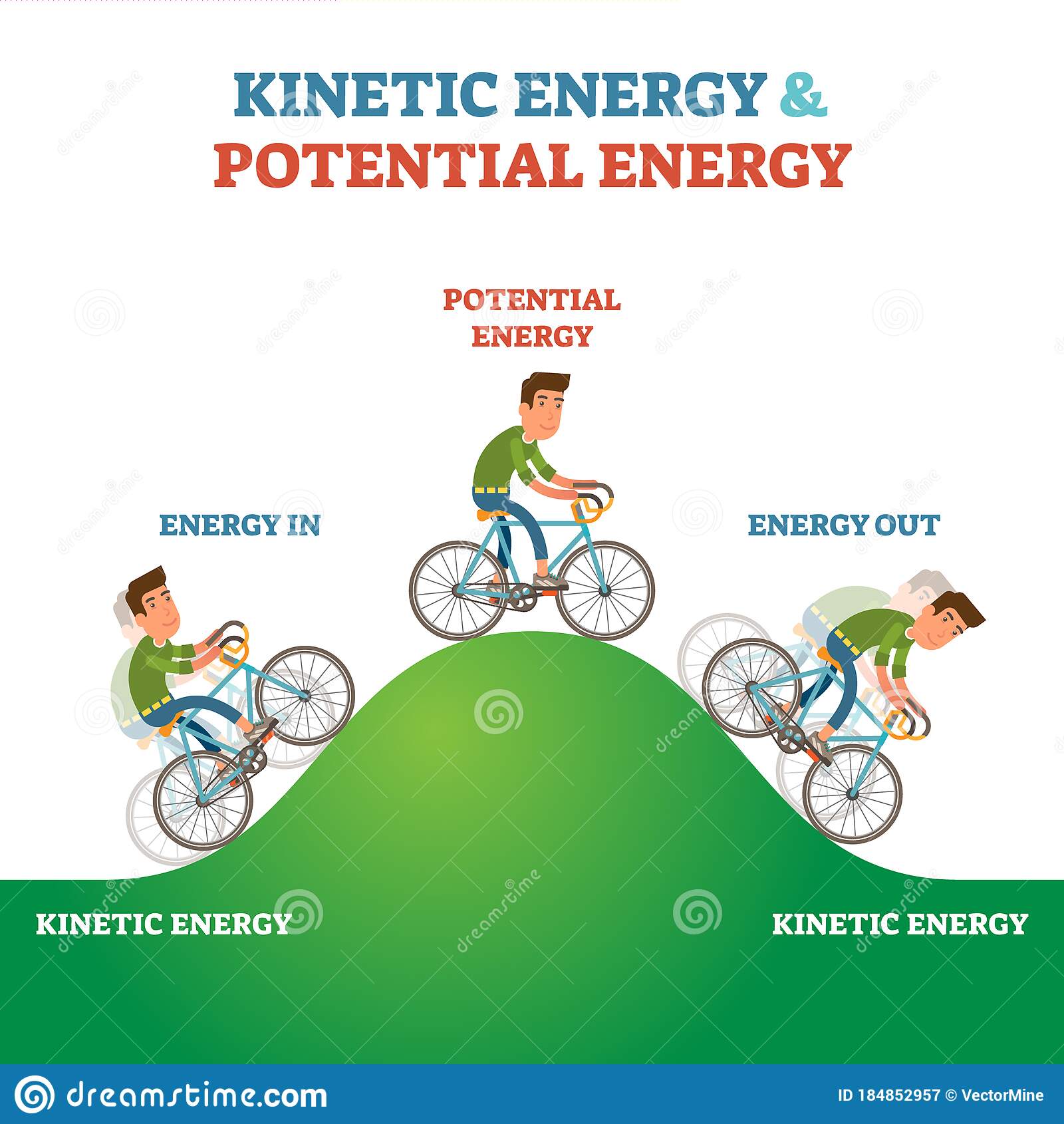
Sometimes an object is pulled or pushed in a particular direction, but its movement is stopped by some other force. In this case, the object is said to have potential energy the potential to move.
Get an update of science stories delivered straight to your inbox.
Transformation Of Mechanical Energy Into Electrical Energy
AC generator transforms the mechanical energy into electrical energy. The most common example of this is the Turbine. A flow of water is used to rotate the turbine and we get electricity from it. The flow of water has kinetic energy which is a type of mechanical energy. This kinetic energy converts into the rotational kinetic energy of the turbine and finally the rotational kinetic energy generates electricity.
Recommended Reading: Infinite Algebra 1 Graphing Inequalities
Measuring Energy Vs Power
Although it is not possible to directly measure energy, the work done can be defined and measured. The methods involves using a calorimeter, which measures the heat absorbed or released in chemical reactions or physical changes, thermometer, which measures temperature or bolometer that is employed to measure the intensity of radiation. Energy generated can be stored whereas power cannot.
Since power is energy per unit of time, in theory it can be calculated after measuring the energy used per second. When calculating the real power consumption of an electrical device, it is essential to measure the voltage applied and the current consumed, taking into account the power that is dissipated in the circuit.
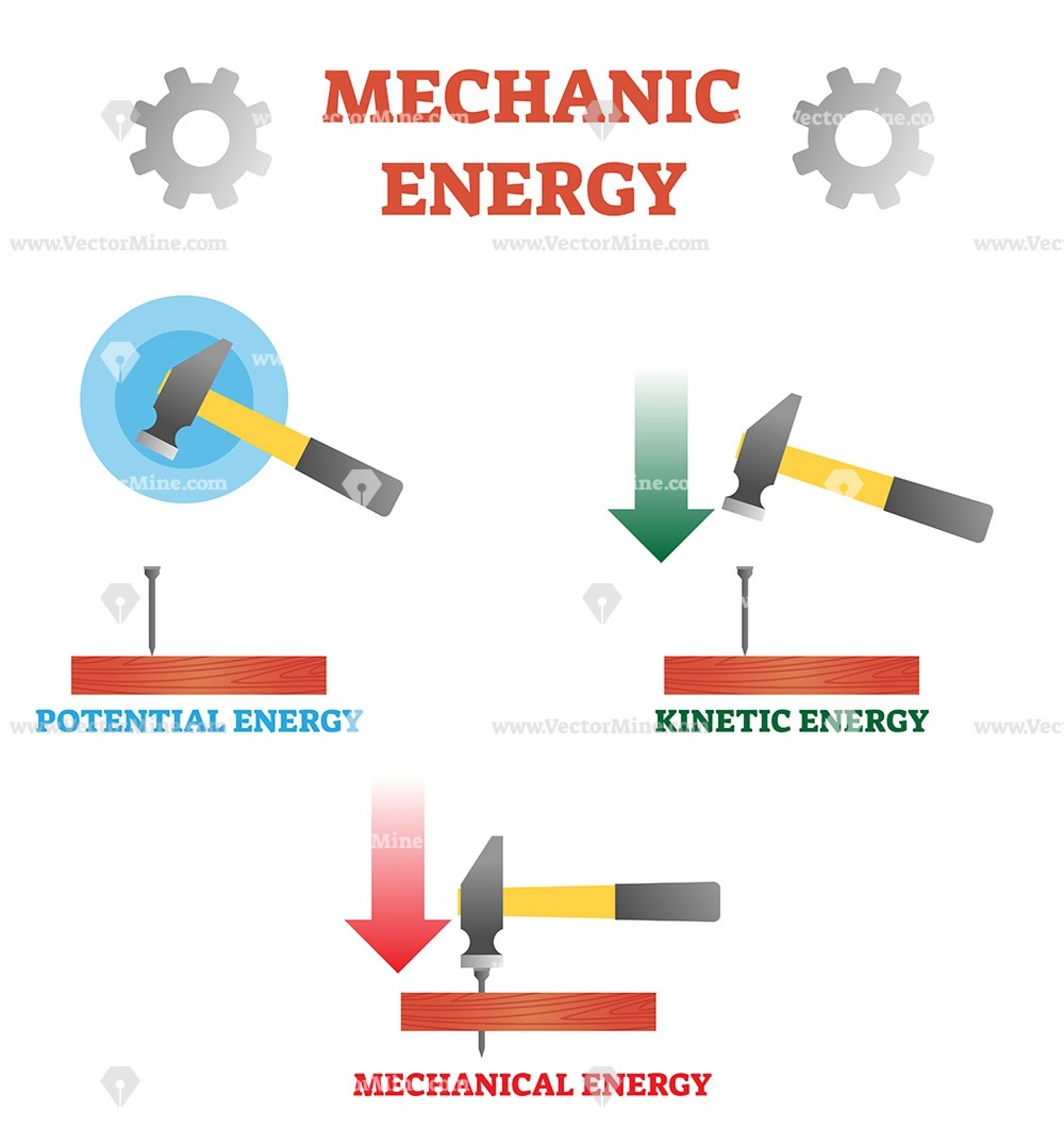
Mechanical Potential Energy In More Detail
The most common types of mechanical potential energy you might learn about include:
- Gravitational potential energy: The energy stored in an object based on its location in a gravitational field. For example, a ball held high above the earth has gravitational potential energy. When released, it will drop as a result.
- Electric potential energy: This is the energy stored in a charged object due to its position in an electric field. For example, the electrons in a circuit will become endowed with a certain amount of electric potential energy due to the battery. When the circuit is connected, this causes the electrons to flow.
- Magnetic potential energy: This is energy stored in an object with magnetic moment due to its location in a magnetic field. Consider when you hold two button magnets near each other and you feel them tugging this is because of the magnetic potential energy.
- Elastic potential energy: This is energy stored in an elastic material. For example, a stretched rubber band has stored energy, as does a compressed spring. When either are released, they will move.
Don’t Miss: What Is Cro In Physics
Energy And Classification Of Energy
In recent days, we often hear about energy. Every invention and civilization is based upon acquiring and effectively using energy. This is possible by the unique property of our Universe, that it can transfer and transform energy, but the total amount is always the same .
One fundamental focus of physics is to investigate energy. Energy in Physics can be generally defined as a scalar quantity associated with the state or condition of one or more objects.
The Formula For Energy:
Kinetic Energy: The energy exists due to the motion of an object is known as Kinetic Energy. For example, a moving van, flowing water, etc.
Where,
| h | Height |
Mechanical Energy: It is the sum total of potential energy and kinetic energy which is the energy associated with the motion & position of any object. Therefore, the formula of mechanical energy will be:
Mechanical Energy = Kinetic Energy + Potential Energy
The law of conservation of energy is one of the basic laws of Physics. It states that In a closed and isolated system from its surroundings, the total energy of the system will be conserved. Thus energy can neither be created nor destroyed, although it can only be transformed from one form to another.
You May Like: What Is Behaviour In Psychology