Theoretical Yield Moles And Grams
To start with, youll need to determine how much reactant you have. Note that the number of reactants you have may be different from the amount in your balanced equation. Afterward, determine the limiting reactant.
Every chemical reaction has a transition state. Derek Barton
Start by using a periodic table to find the atomic weights of the elements that make up the molecules of your reactants. Find this by finding the weight of all the atoms in a single molecule. Now examine your balanced equation and find how the ratio between the reactants is defined. If one mole of a specific reactant results in two moles of a product, the ratio of reactant to the product is 1:2.
Theoretical yield is the amount of product that will be produced by the completed conversion of limiting reactant during a chemical reaction. Photo: bdyczewski via Pixabay, CC0
Now that you have the ratio between reactant and produced product defined, examine how much reactant and product you actually have. If the amount of actual reactant is precisely the same as what was defined in the balanced chemical equation, then the theoretical yield of your reactants is just the amount of product that your equation had determined. You can easily convert the number to grams by multiplying the molecular weight of the product with the number of moles.
How To Find Theoretical Yield
To determine the theoretical yield of any chemical reaction, multiply the number of moles by the molecular weight. Theoretical yield will be calculated in grams because it uses the theoretical yield equation and it is the amount of the expected product. This makes calculating theoretical yield easy.
Now we will solve example with theoretical yield formula to make it more clear. To learn about grams & moles and to calculate their values, use grams to moles calculator.
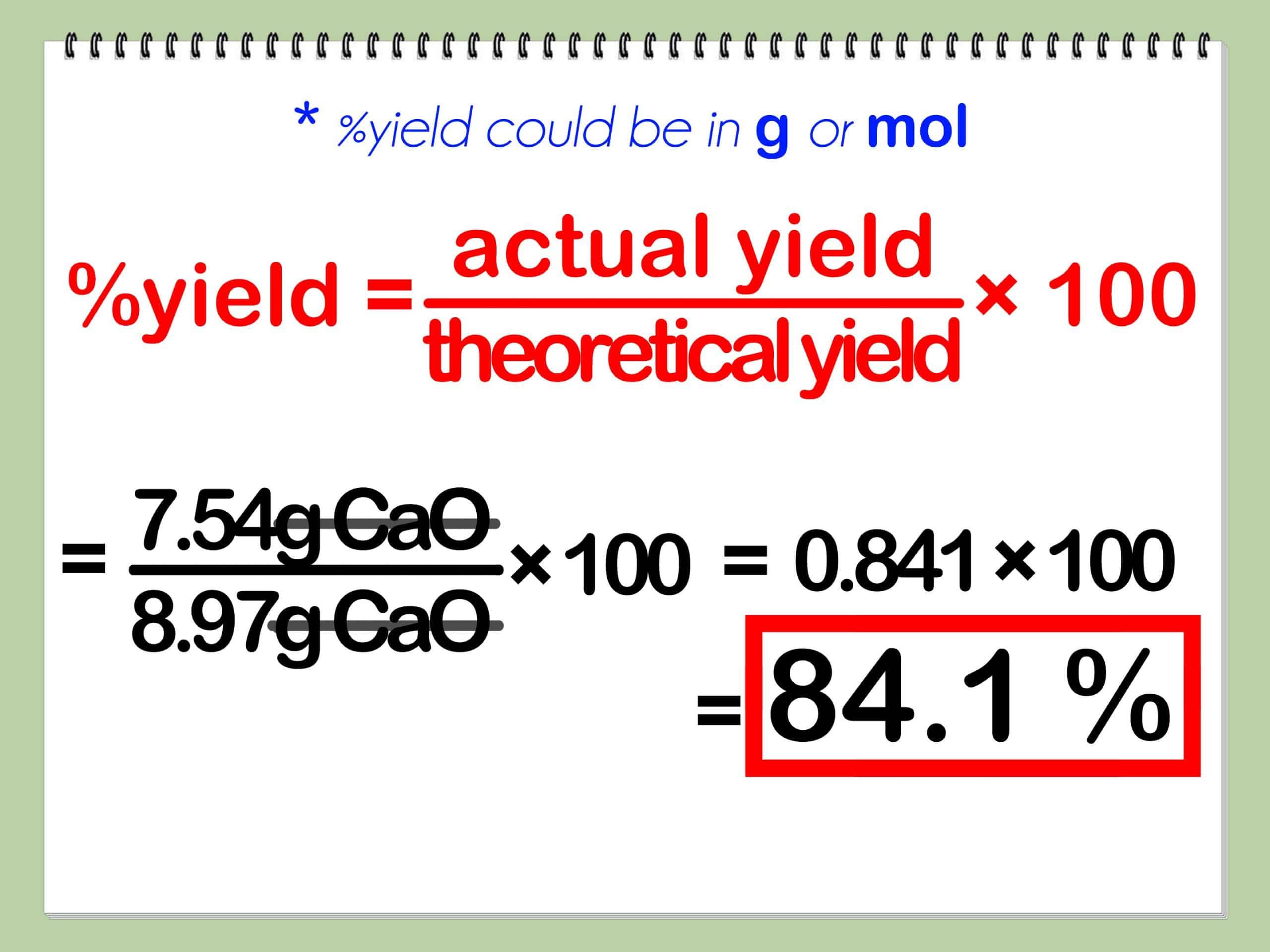
Theoretical Actual And Percent Yields
The percent yield is a comparison between the actual yieldwhich is the weight of the intended product of a chemical reaction in a laboratory settingand the theoretical yieldthe measurement of pure intended isolated product, based on the chemical equation of a flawless chemical reaction, and is defined as,
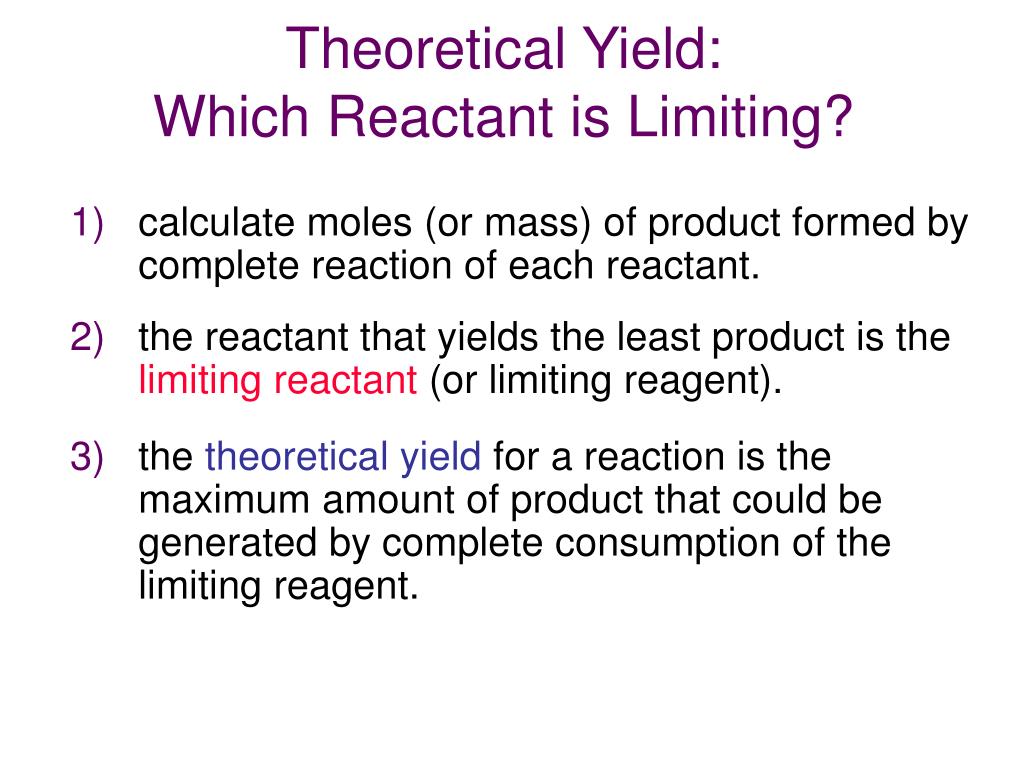
When more than one reactant participates in a reaction, the yield is usually calculated based on the amount of the limiting reactant, whose amount is less than stoichiometrically equivalent to the amounts of all other reactants present. Other reagents present in amounts greater than required to react with all the limiting reagent present are considered excess. As a result, the yield should not be automatically taken as a measure for reaction efficiency.
In their 1992 publication General Chemistry, Whitten, Gailey, and Davis described the theoretical yield as the amount predicted by a stoichiometric calculation based on the number of moles of all reactants present. This calculation assumes that only one reaction occurs and that the limiting reactant reacts completely.
Also Check: Segment Addition Postulate In Geometry
Theoretical Yield Vs Actual Yield Or Percent Yield
Recall that theoretical yield is the theoretical maximum amount of product that will result from a reaction. However, not all reactions are 100% efficient in fact, few are.
Actual yield then is the actual amount of product resulting from a chemical reaction.
The comparison between the theoretical yield and the actual yield is the percent yield. So, percent yield is the ratio between the theoretical and actual yields.
You can use a to calculate this ratio, or you can use the percent yield formula:
percent yield = actual yield / theoretical yield× 100%
Percent yield is equal to the actual yield divided by the theoretical yield, times 100%.
Using this equation its also possible to calculate the theoretical yield if the actual yield and percent yield are known.
theoretical yield = actual yield / percent yield× 100%
Theoretical yield is equal to the actual yield divided by the percent yield, multiplied by 100%.
For Instance 1 Signifies 1 Standard Deviation Away From
. Theoretical Yield Quick Review. The output of a solar installation panel is measured in watts W and indicates the panels theoretical power generation under perfect sunshine and temperature conditions. Easy Steps for All Platforms.
M where 1 M 1 molliter. Write down a balanced chemical equation for the reaction. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield such as how to find theoretical yield as well as the theoretical yield definition and the theoretical yield formulaBefore carrying out any kind of lab work you need to work out what is the theoretical yield so you know how much of your.
Deal with Braces at School. Blurring Your Background in Zoom. Calculate using the following strategy.
Chemistry explains it as the maximum anticipated result. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. The next step is to identify the limiting reactant.
The example above shows a three-step method to synthesise Lacosamide an important medication for epilepsyStep 2 is a stumbling block with the lowest percentage yield of 37. Calculate the Enthalpy of a Chemical Reaction. Molar concentration c is the amount of substance in a certain volume of the substance.
The theoretical yield is the maximum amount of product that can be pro. How does standard deviation look in a normal distribution graph. Bathe Your Pet Rabbit.
Don’t Miss: Algebra Math Problems For 8th Graders
Calculate The Theoretical Yield:
The theoretical yield is the yield you would get if the reaction worked perfectly. That is, if every molecule reacted exactly as it was supposed to, and no material was lost at any stage. The theoretical yield is based on the moles of limiting reagent you started with. Look at the number of moles of limiting reagent and look at the balanced equation. If the reaction takes place consuming the limiting reagent as indicated by the equation, how much product will be produced? This is the theoretical yield.
Example:
Lets consider a simple example first, equation 3 from above. In this example, there is only one reactant 3COH, so this is the limiting reagent . If we started with 1 mol of 3COH, how many moles of 2C=CH2 would we expect for a theoretical yield?
The answer is theoretical yield = 1 mol. The stoichiometry of this reaction is such that every molecule of the limiting reagent gives one molecule of 2C=CH2.
Example:
Now lets consider an example from before. In part 4 above we determined that if we started with 10 mol of ClCH2CH2CH2Cl and 12 mol of NaI in the reaction below that NaI was the limiting reagent. Under these conditions, what is the theoretical yield of ICH2CH2CH2I?
The answer is theoretical yield = 6 mol. It takes two molecules of NaI to make one molecule of ICH2CH2CH2I.
Examples Of Yield Calculations
Time for some examples. Let’s say you are doing a nucleophilic addition reaction, forming hydroxyacetonitrile from sodium cyanide and acetone.
Let’s ignore the solvents underneath the arrow , but also the sodium cation of the sodium cyanide, as it is just a spectator ion. If we react 5 g of acetone with 2 g of cyanide, what is the theoretical yield of hydroxyacetonitrile?
We need to work out the limiting reagent first. As the stoichiometry of both reagents is 1 , we can simply use the mass = molecular weight × mole equation to find this:
Knowing the limiting reagent and its moles means that we know how many moles of the product will form. As the stoichiometry of the product is 1, 0.0769 moles will form.
We can once again use the mass = molecular weight × mole equation to determine the theoretical mass of the product. The molecular weight of hydroxyacetonitrile is 85 g / mol:
mass = 85 × 0.0769 = 6.54 g
Now we know that if we carry out the experiment, we would expect 6.54 g of hydroxyacetonitrile. Not too bad right!
Let’s say you are trying to synthesize acetone to use in the above reaction.
You May Like: How To Pass Math Class
The Limiting Reagent And Excess Reagent
In a chemical reaction, you have both limiting reagents and excess reagents. The limiting reagents are the lesser in numbers and determine how much of a product will result. Therefore, limiting reagents often equals product. Excess reagents are the chemical or chemicals left over, the by-products of a reaction. The limiting reagent is important because for higher effectivity you want a balanced reaction. The ratio of limiting reagent, or limiting reactant and excess reagent, should be as close to 1:1 as possible. The more balanced the solution, the less waste results.
Convert All Amounts Of Reactants And Products Into Moles:
Usually reactants are measured out by volume or mass. You need to know these quantities in terms of moles to do yield calculations. The conversion of volume and mass into number of moles can be done using the density and molecular weight of the material
Mass can be converted to moles using molecular weight. Be sure to include all units in your calculations. It will help you to avoid errors. By insuring that the mass units cancel in the calculation you can be sure you have the calculation setup properly.
Example:
Consider the reaction in equation 3 above. Suppose you used 25.0 g of the reactant 3COH. To convert grams to moles use the molecularweight. So how do you know whether tomultiply or divide by the molecular weight? Answer: look at the units, grams should cancel in the calculationleaving an answer that has units of moles. This is illustrated below.
To convert volume to moles, first convert to massusing density, then convert to moles using molecular weight. Again, be sure to include all units in yourcalculations. It will help you to avoiderrors.
Example:
Againconsider the reaction in equation 3 above. Suppose you used 30.0 mL of the reactant 3COH. First convert this volume into mass usingdensity , then convert grams to moles using the molecular weight. Again, include units and set up yourcalculation so that milliliters and grams cancel in the calculation leaving ananswer that has units of moles. This isillustrated below.
You May Like: How To Graph Physics Data
How To Calculate Theoretical Yield Chemistry
Learn How to Calculate Theoretical Yield Theoretical yield refers to how much product will be produced with given quantities of reactants. Heres how to calculate it.
Before performing chemical reactions, it is helpful to know how much product will be produced with given quantities of reactants. This is known as the theoretical yield. This is a strategy to use when calculating the theoretical yield of a chemical reaction. The same strategy can be applied to determine the amount of each reagent needed to produce a desired amount of product.
Video advice: How to Calculate Theoretical Yields
Introduction to basic organic laboratory equipment and techniques.
How Can I Calculate The Theoretical Yield
I can easily calculate the theoretical yield of a solid or gaseous product using a balanced chemical equation and stoichiometry concept.
But the theoretical yield most times are bigger than the actual yield . This is due to the fact that the efficiency of an experiment may not be 100% because of some practical errors like materials used, machines involves, measurements, etc
Read Also: How Does Physical Geography Affect Military Strategy And Planning
What Is The Theoretical Yield
Chemical equations are very useful not only because they describe how chemical compounds react, which products they form and, partly, how they do so but also because they let us evaluate the performance of any real-life procedure. Imagine you want to produce potassium sulfate, a substance commonly used in fertilizers thanks to its content of both potassium and sulfur. A simple way to form potassium sulfate is by mixing potassium hydroxide, our source of K, with sulfuric acid, as a source of S. This typical acid-base reaction can be described by the following chemical equation:
Thanks to this equation, we know our reaction does not only form the desired product, potassium sulfate, but it also releases water. Furthermore, we can predict that if we mix 2 moles of potassium hydroxide with 1 mol of sulfuric acid, we will produce 1 mol of potassium sulfate and 2 moles of water. This is true, of course, in an ideal world. In reality, if you perform the reaction, you will probably notice these amounts do not necessarily match what you get.
This can occur due to your reagents not being entirely pure, or due to the presence of additional contaminants that get in the way of your reaction. Furthermore, there are fundamental conditions that can still cause a perfectly clean reaction not to proceed to completion, thus altering the expected amount of products. Lets try to understand how.
What Is Actual Yield
Actual yield is the real quantity of a product resulting from a chemical reaction. Where the theoretical yield is the amount that may be obtained by a reaction and assumes a 100% result of a product, the actual yield is typically less. This is because few reactants proceed to completion or not all the products can be obtained. If the resulting product needs to be filtered, or some sticks to the inside of the beaker, these variables affect actual yield. The formula to determine actual yield is simple: you multiply the percentage and theoretical yield together.
You May Like: What Are The Possible Benefits Of Studying Biology
Three: Find The Moles Of Limiting Reagent
After you have found the limiting reagent, youll need to calculate how many moles of the limiting reagent will be in the reaction. You can use the following formula to calculate this:
limiting reagent = mass / molecular weight × stoichiometry
So, the moles of limiting reagent are equal to the mass of limiting reagent in grams divided by its molecular weight in g/mol, multiplied by the limiting reagent stoichiometry in the reaction, as found above when balancing the equation.
Multiply By 100 To Convert To A Percentage
To find the percent yield and calculate a full percentage, take the decimal results from the above step and multiply them by 100. This is the percent yield of a chemical reaction and helps to make the most product with the least waste while serving to indicate the efficiency of the method.
Read more:15 Top Chemistry Degree Jobs
Read Also: What Does Annex Mean In Math
S In Finding Percent Yield
Calculating Percent Yield In chemistry, percent yield is a way of gauging the completeness of a reaction. Percent yield compares the actual yield of a compound in a reaction to the theoretical yield of that compound. The theoretical yield assumes that all of the limiting reagent was consumed in a compound. In other words, the reaction took place completely. You must divide the grams of your actual yield by the grams of the theoretical yield and multiply by 100 in order to obtain percent yield. Determine the Limiting Reagent Calculate the molar mass of all the compounds in the chemical reaction. The molar mass is the sum of the atomic mass of each atom in a compound. For example, the molar mass of water is 18 grams: 2 grams of hydrogen plus 16 grams of oxygen. Divide the grams of the compounds by their molar masses. This will give you the number of moles of each compound in the experiment. For example, if you initially used 36 grams of water, 36 divided by 18 grams per mole yields 2 moles of water. Compare the moles of reactants in your experiment to the theoretical number of moles.
Express Mass Of The Reactants In Terms Of Moles
Here, we have to convert and express the given mass of Na and Cl2 in terms of moles. We have learned that molar mass is the mass of 1 mole of a substance. The molar mass of Na is given to be 22.99 g/mole, and the molar mass of Cl2 is given to be 70.90 g/mole.
We have to calculate the number of moles present in 4 grams of Na and 15 grams of Cl2. We can do so by using this formula:
Number of moles of a substance = mass of the reactant ÷ molar mass of the reactant
Number of moles of Na = amount of Na in the reaction ÷ molar mass of Na
Number of moles of Na = 4 grams ÷ 22.99 g/mole
Number of moles of Na = 0.17 moles of Na
Similarly:
Number of moles of Cl2 = 15 grams ÷ 70.90 g/mole
Number of moles of Cl2 = 0.21 moles of Cl2
Read Also: What Is The Paradox Of Choice Psychology
Limiting Reactant And Percent Yield
The limiting reactant of a balanced chemical equation is identified to determine theoretical yield. Because the limiting reactant isnt found in abundance, the reaction cant continue once used up.
To find the limiting reactant, the below points are to be remembered:
- If the quantity of reactants is given in moles, convert the results to grams.
- In grams per mole, divide the mass of the reactant by its molecular weight.
- Alternatively, we can multiply the amount of a reactant solution in millilitres by its density in grams per millilitre for a liquid solution. Then divide the result by the molar mass of the reactant.
- Multiply the mass obtained by the number of moles of the reactant in the balanced equation using either technique.
- Now we know how many moles each reactant has. To determine which is accessible in excess and which will be used up first , compare this to the molar ratio of the reactants.
To calculate the percent yield, first establish the amount of product that should be produced using stoichiometry. This is the maximum amount of product made with reactant amounts available. When a reaction is carried out in the lab, the actual yield is the amount of produced product. The percent yield is the percentage difference between the actual and theoretical yields.
Percent Yield = Mass of Actual Yield / Mass of Theoretical Yield x 100 percent