Physical And Chemical Properties
Cellulose acetate has a melting point of 306 ° C, a density that ranges from 1.27 to 1.34, and has an approximate molecular weight of 1811.699 g / mol.
It is insoluble in various organic components such as acetone, cyclohexanol, ethyl acetate, nitropropane, and ethylene dichloride.
Of the products that contain cellulose acetate, flexibility, hardness, tensile strength, not being attacked by bacteria or microorganisms and their impermeability to water are valued.
However, the fibers present dimensional changes according to extreme variations in temperature and humidity, although the fibers resist temperatures up to 80 ° C.
Key Difference Between Amylose And Cellulose With Table
What is the difference between amylose and cellulose?
Starch is a carbohydrate which is grouped as a polysaccharide. A combination of several monosaccharide units through glycosidic bonds results in polysaccharides. These polysaccharides are polymers and monosaccharides are monomers.
The core difference between amylose and cellulose is that amylose is a stored form of polysaccharide where D-glucose molecules are linked via -1, 4-glycosidic bond to form a linear structure while cellulose is a structural polysaccharide where D-glucose molecules are linked via glycosidic bonds to form a linear structure.
Importance To Human Diet
Despite the fact that humans cannot digest cellulose , cellulose is nonetheless a very important part of the healthy human diet. This is because it forms a major part of the dietary fiber that we know is important for proper digestion. Since we cannot break cellulose down and it passes through our systems basically unchanged, it acts as what we call bulk or roughage that helps the movements of our intestines. Among mammals, only those that are ruminants can process cellulose. This is because they have special bacteria and microorganisms in their digestive tracts that do it for them. They are then able to absorb the broken-down cellulose and use its sugar as a food source. Fungi are also able to break down cellulose into sugar that they can absorb, and they play a major role in the decomposition of wood and other plant material.
May 29 2018
Recommended Reading: What Does Abiotic Mean In Biology
How Is Cellulose Important For Humans
Cellulose is a polysaccharide that makes 30% of the plant cell wall. It helps in connecting cells to form tissues and signals the cells to grow and divide. Humans cannot digest cellulose. However, it is consumed in the diet as fibre. Fibre helps the digestive system to keep the food moving through the gut and moves the waste out of the body.
How Cellulose Is Used In Food
Fiber Supplement: With rising awareness about fiber intake, cellulose has become one of the most popular food additives. Adding cellulose to food allows an increase in bulk and fiber content without a major impact on flavor. Since cellulose binds and mixes easily with water, it is often added to increase the fiber content of drinks and other liquid items when the gritty texture of regular fiber supplements would be undesirable.
Calorie Reducer: Cellulose provides a lot of volume or bulk of food but because it is indigestible to humans, it has no caloric value. For this reason, cellulose has become a popular bulking agent in diet foods. Consumers who eat foods with high cellulose content feel full physically and psychologically without having consumed many calories.
Thickening/Emulsifying: The gelling action of cellulose when combined with water provides both thickening and stabilizing qualities in the food to which it is added. Cellulose gel acts similarly to an emulsion, suspending ingredients within a solution and preventing water from separating out. Cellulose is often added to sauces for both the thickening and emulsifying action.
The thickening power of cellulose also allows for more air to be whipped into products like ice cream, or whipped topping. Cellulose allows for the production of thick and creamy food items without the use of as much fat.
Also Check: What Is Elastic Force In Physics
Extraction And Characterization Of Cellulose
Cellulose is one of the most abundant biomass materials in nature possessing some promising properties. In our work, let us look into the various procedures involved in extracting cellulose from different sources. The sources available are many which can be broadly classified into Agro-waste, Domestic-waste . The processes involved include usual chemical procedures like alkaline extraction, bleaching, acid hydrolysis and chlorination. The final products can be characterized by using techniques like thermogravimetric analysis , infrared spectroscopy , X-ray diffraction , differential scanning calorimetry and scanning electronic microscopy . Purified cellulose can be obtained successfully using the above mentioned procedures. In our study, we will be concentrating on the extraction of cellulose from agricultural residues and plants .
Figure 4.
How It Is Made
Cellulose is synthesized in plants and some microorganisms through the process known as photosynthesis. In that process, carbon dioxide and water are combined in a complex series of reactions to produce glucose and oxygen . Glucose molecules are then linked to each other to from successively larger and more complex molecules, eventually resulting in the formation of cellulose.
Commercially, most cellulose is extracted from wood by one of two methods, the kraft process or the steam explosion process. The product of these reactions is wood pulp, which consists primarily of cellulose. In the kraft process, wood chips are treated with a solution of sodium hydroxide and sodium sulfide at temperatures of about 175°C for two to six hours. This process usually results in a yield of about 40 to 45 percent wood pulp. The pulp is then treated with a bleaching agent, such as calcium or sodium hypochlorite 2 or NaClO) or chlorine dioxide to remove the color of lignin and other impurities.
Also Check: What Is The Molecular Geometry Of Ccl4
Oligomers And Structure At The Nanoscale Level
The oligomers of cellulose, which are essentially insoluble at the octamer level, have been the subject of a number of investigations because their secondary and tertiary structures seem to converge to structures similar to those of cellulose II. The X-ray powder patterns are quite similar to those of cellulose II,123 and the vibrational spectra, both Raman and infrared, converge to that of cellulose at the tetramer.48 The high-resolution solid-state 13C NMR also converge to that of cellulose II, although at the tetramer the resonance of the anomeric carbon on the reducing end is still distinct.58,69
In one of the studies cited,58 a multidisciplinary approach was used to explore the structures and led to the conclusion that the molecules of the celotetraose were arranged in an antiparallel manner, while another, based on X-ray diffractometry, concluded that a parallel structure is equally probable.123 These studies also indicated that the dihedral angles of the glycosidic linkage departed systematically from those of a twofold helix. As noted earlier, the recent results of Maurer and Fengel105 point to a parallel alignment of the molecular chains in the structure of cellulose II. Thus, in spite of many studies, the structures of both cellulose II and the oligomers remain the subject of many unanswered questions.
Andrew N. Amenaghawon, … Heri Septya Kusuma, in, 2021
Is Cellulose Branched Or Unbranched
Cellulose is the main polysaccharide used for structural function in plants. This is one of the most common organic compounds found on the planet, obviously. Cellulose is an unbranched glucose residue polymer put together via beta-1,4 connections, which enables the molecule to form long, straight chains.
Learn more about the structure and properties of n from the expert faculties at BYJUS.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: What Is The Molecular Geometry Of Ccl4
What Is Cellulose Write Short Notes On It
CELLULOSE: Cellulose is a polymer of glucose which has the molecular formula n with n ranging from 500 to 5,000, depending on the source. -glucose units are linked at C1-C4 in a linear chain. The beta-glycoside bonds allow the chains to stretch out, and the chains are stabilized by intramolecular hydrogen bonds. Hemicellulose: Hemicellulose is a low molecular weight, branched, amorphous polymer. Hence, compared to cellulose, hemicellulose is structurally weak and is easily hydrolyzed by dilute acid or base and enzymes. Hemicelluloses contain many D-pentose sugars, with xylose being the major component. Mannose and mannuronic acid are also present, and similarly galactose and galacturonic acid.
Was this answer helpful?
What Is Cellulose Facts And Functions
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Cellulose is an organic compound and the most abundant biopolymer on Earth. It is a complex carbohydrate or polysaccharide consisting of hundreds to thousands of glucose molecules, linked together to form a chain. While animals don’t produce cellulose, it is made by plants, algae, and some bacteria and other microorganisms. Cellulose is the main structural molecule in the cell walls of plants and algae.
Read Also: Geometry Segment Addition Postulate Worksheet
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
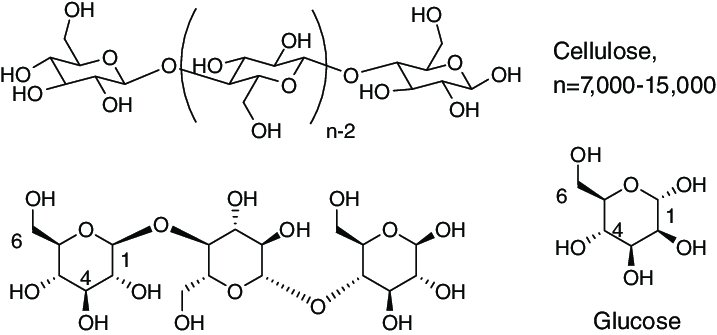
Cellulose Esters And Ethers
The hydroxyl groups of cellulose can be partially or fully reacted with various reagents to afford derivatives with useful properties like mainly cellulose esters and cellulose ethers . In principle, although not always in current industrial practice, cellulosic polymers are renewable resources.
Ester derivatives include:
Cellulose for industrial use is mainly obtained from wood pulp and from cotton.
Also Check: Practice And Homework Lesson 1.7 Answers
Cellulose Network In Plants Cell Wall
Understanding the arrangement of cellulose microfibrils and polysaccharide matrix in the cell wall of plants is also important.
We have studied earlier that as the cellulose chains are synthesized, they are exported out of the cell into the cell wall. Here the cellulose chains are arranged in parallel fashion forming hydrogen bonds among themselves. This results in the formation of cellulose microfibrils.
Polysaccharide matrix is formed when other sugar molecules interact with these cellulose microfibrils. In the primary cell wall of plants, glucans and arabinoxylans are the two major components of the polysaccharide matrix. These polysaccharides interact with one another and form a network among the cellulose microfibrils. This network is strengthened by cross-links formation. These cross-links are formed when arabinoxylan residues react with acids like ferulic acid and diferulic acid . Due to this reason, it is also said that the polysaccharide matrix is made up of acidic polysaccharides.
In addition to the cellulose microfibrils and polysaccharide matrix, the primary cell wall also contains cross-linking polysaccharides. These polysaccharides cross-link the cellulose microfibrils to form a complex network. Most important of these cross-linking polysaccharides is hemicellulose. It is a derivative of cellulose and will be discussed briefly towards the end of this article.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Recommended Reading: Is Paris Jackson Biological
Introduction: Starch And Cellulose
Polysaccharides are the most abundantly available in nature among carbohydrates and perform a variety of functions, such as energy storage or as components of plant cell walls.
Polysaccharides are very large polymers made up of tens to thousands of monosaccharides, linked by glycosidic linkages.
Ccommon polysaccharides: Starch, glycogen, and cellulose.
Heteropolymers can include, in addition to monosaccharides, sugars, amino sugars, or non carbohydrate substances.
Heteropolymers are common in nature and are non reducing carbohydrates .
The Structure Of Cellulose
Cellulose is usually described by chemists and biologists as a complex carbohydrate . Carbohydrates are organic compounds made up of carbon, hydrogen, and oxygen that function as for living things. Plants are able to make their own carbohydrates that they use for energy and to build their cell walls. According to how many atoms they have, there are several different types of carbohydrates, but the simplest and most common in a plant is glucose. Plants make glucose to use for energy or to store as starch for later use. A plant uses glucose to make cellulose when it links many simple units of glucose together to form long chains. These long chains are called polysaccharides (meaning “many sugars”
and pronounced pahl-lee-SAK-uh-rydes), and they form very long molecules that plants use to build their walls.
It is because of these long molecules that cellulose is insoluble or does not dissolve easily in water. These long molecules also are formed into a criss-cross mesh that gives strength and shape to the cell wall. Thus while some of the food that a plant makes when it converts light energy into chemical energy is used as fuel and some is stored, the rest is turned into cellulose that serves as the main building material for a plant. Cellulose is ideal as a structural material since its fibers give strength and toughness to a plant’s leaves, roots, and stems.
Recommended Reading: Who Are Paris Jackson’s Biological Parents
Structure Of Cellulose N
Structure of Cellulose n
In high temperature, It can be broken down into glucose by treating with concentrated minerals acids. It is more crystalline when compared to starch. But starch goes from crystalline to amorphous transition in 60-70 degrees but cellulose, on the other hand, requires 320 degrees and a pressure of 25 Megapascal.
How Cellulose Is Arranged In Plant Cell Walls
Like human bone, plant cell walls are composed of fibrils laid down in a matrix, or background material. In a cell wall, the fibrils are cellulose microfibrils, and the matrix is composed of other polysaccharides and proteins. One of these matrix polysaccharides in cell walls is pectin, a substance that, when heated, forms a gel. Pectin is the substance that cooks use to make jellies and jams.
The arrangement of cellulose microfibrils within the polysaccharide and protein matrix imparts great strength to plant cell walls. The cell wall of plants performs several functions, each related to the rigidity of the cell wall. It protects the interior of the plant cell, but also allows the circulation of fluids within and around the cell wall. The cell wall also binds the plant cell to its neighbors. This binding creates the
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers
Cellulose And Plant Cells
Since cellulose is the main building material out of which plants are made, and plants are the primary or first link in what is known as the food chain , cellulose is a very important substance. It was first isolated in 1834 by the French chemist Anselme Payen , who earlier had isolated the first enzyme. While studying different types of wood, Payen obtained a substance that he knew was not starch , but which still could be broken down into its basic units of glucose just as starch can. He named this new substance “cellulose” because he had obtained it from the cell walls of plants.
Human Uses Of Cellulose
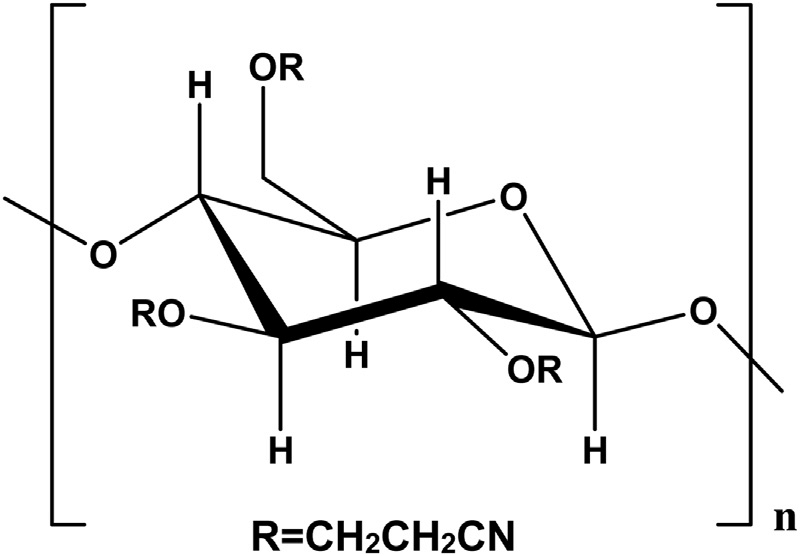
Cellulose is one of the most widely used natural substances and has become one of the most important commercial raw materials. The major sources of cellulose are plant fibers and, of course, wood . Since cellulose is insoluble in water, it is easily separated from the other constituents of a plant. Cellulose has been used to make paper since the Chinese first invented the process around a.d. 100. Cellulose is separated from wood by a pulping process that grinds woodchips under flowing water. The pulp that remains is then washed, bleached, and poured over a vibrating mesh. When the water finally drains from the pulp, what remains is an interlocking web of fibers that, when dried, pressed, and smoothed, becomes a sheet of paper.
Don’t Miss: Can Work Be Negative Physics
Chemical Structure And Properties
Cellulose forms via -glycosidic bonds between D-glucose units. In contrast, starch and glycogen form by -glycosidic bonds between glucose molecules. The linkages in cellulose make it a straight chain polymer. The hydroxyl groups on the glucose molecules form hydrogen bonds with oxygen atoms, holding the chains in place and conferring high tensile strength to the fibers. In plant cell walls, multiple chains bond together to form microfibrils.
Pure cellulose is odorless, flavorless, hydrophilic, insoluble in water, and biodegradable. It has melting point of 467 degrees Celsius and can be degraded into glucose by acid treatment at high temperature.
Key Difference Cellulose Vs Hemicellulose
Cellulose and hemicellulose are two types of natural polymers that are mainly found in the plant cell walls and are important components of natural lignocellulosic materials. But, these two components are different in the chemical composition and the structure. The key difference between cellulose and hemicellulose is that cellulose is an organic polysaccharide molecule whereas hemicellulose is a matrix of polysaccharides.
Don’t Miss: Prince Jackson’s Biological Father