Preference Over One Another
As an example, when potassium permanganate dissolves in water, it loses five electrons. These are the active species of this compound.
Molar wt of KMnO4 = 158.04
No of active species = 5
Gram equivalent wt = Mol. wt / n
Hence, G.eq = 31.6
Thus, it can be concluded that molarity is easier to calculate as compared to normality . The reason is that normality involves the number of active species , which can only be known after studying its behavior in a reaction.
Calculating The Electrons With Ions Present
How Can You Use This
Lets summarize what weve discussed so far:
Up to Z = 20 , the rule correctly predicts:
- Orbital energy levels
The order of occupancy of the orbitals
- The physical meaning of the rule is related to the size and shape of a given orbital.
For Z > 20 :
- The rule is not able to correctly predict orbital energy levels.
- Even when we know the orbital energies, this knowledge is not sufficient to predict the order of filling. Other factors, such as d vs. s electron repulsions must be considered. .
- Although its physical meaning is no long sufficient, the rule still correctly predicts the order of filling. Except where it doesnt and we invoke exceptions.
The first point to be taken from this is that the rule is a model and that it works until it doesnt. If you choose to teach it as a model and connect it to some of the physical meaning discussed above, its a great example of how models can be both useful and also fail.
The story outlined above has the potential to be much more fulfilling for your students than Memorize the diagram, learn to use it, and youre guaranteed to get the right answer. But its a tough story to tell by just waving your hands. You need a model to tell it, and the model needs the following features:
References:
Also Check: Similar Math Definition
The Principal Quantum Number
The principal quantum number, \, designates the principal electron shell. Because n describes the most probable distance of the electrons from the nucleus, the larger the number n is, the farther the electron is from the nucleus, the larger the size of the orbital, and the larger the atom is. n can be any positive integer starting at 1, as \ designates the first principal shell . The first principal shell is also called the ground state, or lowest energy state. This explains why \ can not be 0 or any negative integer, because there exists no atoms with zero or a negative amount of energy levels/principal shells. When an electron is in an excited state or it gains energy, it may jump to the second principle shell, where \. This is called absorption because the electron is “absorbing” photons, or energy. Known as emission, electrons can also “emit” energy as they jump to lower principle shells, where n decreases by whole numbers. As the energy of the electron increases, so does the principal quantum number, e.g., n = 3 indicates the third principal shell, n = 4 indicates the fourth principal shell, and so on.
If n = 7, what is the principal electron shell?
Example \
If an electron jumped from energy level n = 5 to energy level n = 3, did absorption or emission of a photon occur?
- Answer
-
Emission, because energy is lost by release of a photon.
The Orbital Angular Momentum Quantum Number
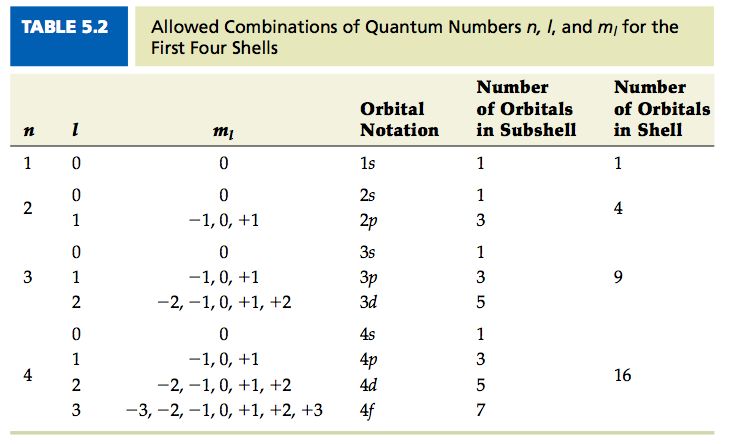
The orbital angular momentum quantum number \ determines the shape of an orbital, and therefore the angular distribution. The number of angular nodes is equal to the value of the angular momentum quantum number \. Each value of \ indicates a specific s, p, d, f subshell The value of \ is dependent on the principal quantum number \. Unlike \, the value of \ can be zero. It can also be a positive integer, but it cannot be larger than one less than the principal quantum number ):
Example \
If \, what are the possible values of \?
- Answer
-
Since \ can be zero or a positive integer less than ), it can have a value of 0, 1, 2, 3, 4, 5 or 6.
Example \
If \, how many angular nodes does the atom have?
- Answer
-
The number of angular nodes is equal to the value of l, so the number of nodes is also 4.
Read Also: Elastic Force Physics
Exercise : Calculating The Mean
The sample mean is the average value for a finite set of replicatemeasurements on a sample. It provides an estimate of the population mean for the sample using the specific measurement method. The sample mean, denoted , is calculated using the formula:
Suppose we use atomic absorbance spectroscopy to measure the total sodium content a can of soup we perform themeasurement on five separate portions of the soup, obtainingthe results 108.6, 104.2, 96.1, 99.6, and 102.2 mg. What isthe mean value for the sodium content of the can of soup?
You have already used the relevant Excel functionsfor this calculation in . Set up a new worksheet and calculatethe mean value, using the COUNT and SUM functions, and the AVERAGEfunction you should get the same values.
Skip to Reporting Results
How To Find Normality
Normality is one of the concentration units of solutions in stoichiometry. It is defined as the gram equivalents of solute, dissolved per liter of a solution. Gram equivalents are the mass of a substance that can produce one mole of chemically active species in solutions.
For example, 1 normal solution of sulphuric acid
Sulphuric acid has a molar mass of 98 g/mol. It means that if its 98g are diluted to make a liter of solution, its normality will be 2N . Therefore, in order to make a 1N solution,
49 grams of H2SO4 One mole of hydrogen ions
Thus, one gram equivalents of sulphuric acid = 49 grams.
In order to calculate the normality of a given solution, chemists titrate it against a counter solution with a known concentration. Acid and bases produce hydrogen ions H+ and hydroxyl OH ions as active species. In salts, these species are electrons.
Normality = Number of Gram equivalents / Liter of solution
Gram equivalent= Molar wt / active species
Units of Normality
When gram equivalent weight is divided per liter of solution, the units used are g.eq/ liter or just N.
Equivalent weight in normality
Gram equivalent weight is the mass of a substance that produces one mole active species . The nature of active species depends on the type of reactions, taking place due to counter-active species present inside solutions.
Don’t Miss: Geometry Chapter 10 Test Form B Answers
When To Use Normality
There are specific circumstances when it’s preferable to use normality rather than molarity or other unit of concentration of a chemical solution.
- Normality is used in acid-base chemistry to describe the concentration of hydronium and hydroxide . In this situation, 1/feq is an integer.
- The equivalence factor or normality is used in precipitation reactions to indicate the number of ions that will precipitate. Here, 1/feq is once again and integer value.
- In redox reactions, the equivalence factor indicates how many electrons can be donated or accepted by an oxidizing or reducing agent. For redox reactions, 1/feq may be a fraction.
Population Versus Sample Mean & Standard Deviation:
If we make only a limited number of measurements , some will be closer to the true value than others. This is becausethere can be variations in the amount of chemical beingmeasured and in the actual measurement itself
This variability contributes to dispersion inthe measured values the greater the variability , the greater thelikelihood that all the measured values may differsignificantly from the true value.
To adequately take this variability into account anddetermine the actual dispersion , we would have to obtain allpossible measurement values in other words,make an infinite number of replicate measurements. This would allow us todetermine the population mean and standarddeviation, and
This is hardly practical, for a number of reasons!The general approach is therefore to perform a limitednumber of replicate measurements . This allows us tocalculate the sample mean and standard deviation, and s.
The sample mean, standard deviation, and variance provide estimates of the population values forlarge numbers of replicates , these approachthe population values.
Skip to Reporting Results
Recommended Reading: Eoc Fsa Warm Ups Answers
Rules To Finding Number Of Protons Neutrons And Electrons
# of protons = atomic number
# of neutrons = mass number atomic number
# of electrons = atomic number charge
Thats it!
Great, lets apply the rules to some examples.
# of protons = 17
# of neutrons = 37 17 = 20
# of electrons = 17 0 = 17
# of protons = 16
# of neutrons = 32 16 = 16
# of electrons = 16 = 18
Electronic Configuration Of Krypton
Figure 3. the electron configuration of krypton.
How is the size of the orbital related to its energy? Recall that the potential energy of attraction between protons and electrons, which have opposite charges, depends on the distance between them: the closer an electron gets to the protons in the nucleus, the lower its energy will be. Compare the sizes of the 1s and 4s orbitals . Because the 1s orbital is smaller, the average distance of an electron to the nucleus will be smaller than that of the electrons in the 4s orbital. Thats the connection the higher n is, the higher the energy of the orbital.
What about the l in the rule? As mentioned above, l, the angular momentum quantum number, determines the shape of an orbital. In all orbitals for which n > 1, there are areas, called nodes, in which it is extremely unlikely to find an electron. There are two types of nodes: radial and planar . Figure 4 illustrates the radial node in a 2s orbital and a planar node in a 2p orbital . Note that radial node does not cross the nucleus, whereas planar nodes do. s orbitals contain only radial nodes. All other orbitals contain both radial and planar nodes.
Don’t Miss: What Are Two Types Of Elastic Forces
Calculation Of Normality By Acid
It is really important to choose the solution of counteractive species . These chemical species are used as an indirect measure of the normality of a given solution. For this purpose, volumes of both counter-active species along with the normality of the standard solution are required.
N1V1 =N2V2
- Normality of first chemical
- Volume of first chemical
- Normality of second chemical
- Volume of second chemical
What Is The Spin Of An Electron
To learn more about quantum numbers and their uses in writing electron configurations, download the BYJUS mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
You May Like: Mm Stands For In Chemistry
Calculating Protons Electrons And Neutrons
Solved Question For You
Q: How can one differentiate Normality and Molarity?
Ans: Morality is the number of moles of solute per litre of solution, whereas Normality is the number of grams equivalent of solute per litre of solution.
Also, Molarity is a measurement of the moles in the total volume of the solution, whereas Normality is a measurement of the gram equivalent in relation to the total volume of the solution.
Don’t Miss: Who Is Khloe Kardashian’s Real Father
Problem Solving Using Moles Mass And Molar Mass
The Problem:
Chris the Chemist has an impure sample of calcium carbonate.The mass of the impure sample is 0.1250 kg and it is composed of 87.00% calcium carbonate.Before Chris can use this calcium carbonate in a chemical reaction, Chris needs to know the amount, in moles, of calcium carbonate present in this sample.
Calculate the amount of calcium carbonate in moles present in this impure sample of calcium carbonate.
Solving the Problem using the StoPGoPS model for problem solving:
| STOP! |
Worked Examples Of Calculating Mass Moles Molar Mass
In each of the worked examples below, you will be asked to calculate either the moles, mass, or molar mass of a pure substance.
To answer each question correctly you will need to:
Read Also: Are Michael Jackson’s Kids Biologically His
The Electron Spin Quantum Number
Unlike \, \, and \, the electron spin quantum number \ does not depend on another quantum number. It designates the direction of the electron spin and may have a spin of +1/2, represented by, or 1/2, represented by . This means that when \ is positive the electron has an upward spin, which can be referred to as “spin up.” When it is negative, the electron has a downward spin, so it is “spin down.” The significance of the electron spin quantum number is its determination of an atom’s ability to generate a magnetic field or not. /Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electron_Spin” rel=”nofollow”> Electron Spin.)
Example \
List the possible combinations of all four quantum numbers when \, \, and \.
- Answer
-
The fourth quantum number is independent of the first three, allowing the first three quantum numbers of two electrons to be the same. Since the spin can be +1/2 or =1/2, there are two combinations:
- \, \, \, \
- \, \, \, \
Example \
-
No, if the value of \ is positive, the electron is “spin up.”
How To Determine The Value For N In The Gibbs Free Energy And Redox Potential Equation
I am having a problem with this equation for redox potentials
$$\Delta G = -nFE_\mathrm$$
In this equation I never am totally sure about what the value of $n$ should be, for example for the reaction shown below, would the $n$ be 2 electrons or 1 electron? Personally, I think it should be 2 electrons because that is the number of mol of electron under the simplest whole number ratio.
\begin\ce\\\ce\\\end
I think this is a different matter than in the already answered question “Does the relationship equation between standard cell potential and equilibrium constant violate potential’s intensive properties?” Because in that Nernst equation the change in $n$ would be balanced by the change in the equilibrium constant. I assume that you are insinuating that there is one of the variables here which will correct for the increase in $n$. I don’t see which would, as $E_\mathrm$ should be independent of quantity and $F$ is a constant.
The accepted answer is not correct, the number $n$ is not equal to 2 simply because 2 is the lowest common multiple of 2 and 1. Furthermore $n$ is not the “number of moles of electrons”, which would have units of $\pu$ $n$ is in fact dimensionless.
In fact the number $n$ can be anything you like, and it depends on how you combine the two half-equations.
To use a different example consider the half-reactions ” rel=”nofollow”> Wikipedia)
$$\begin\ce & E_1 & = \pu \tag \\\ce & E_2 & = \pu \tag \end$$
$$\ce \tag$$
$$\begin\ce \tag \\\ce \tag\end$$
$$\ce \tag$$
Recommended Reading: Steve Harvey’s Biological Children