Limiting Reactants In Chemistry
We can use this method in stoichiometry calculations. Again, if we’re given a problem where we know the quantities of both reactants, all we need to do is figure out how much product will be formed from each. The smaller of these quantities will be the amount we can actually form. The reactant that resulted in the smallest amount of product is the limiting reactant. Let’s see an example:
Example: Using the equation 2 H2 + O2 ? 2 H2O, determine how many moles of water can be formed if I start with 1.75 moles of oxygen and 2.75 moles of hydrogen.
You’ve Got Problems
Problem 2: Using the following equation, determine how much lead iodide can be formed from 115 grams of lead nitrate and 265 grams of potassium iodide:
Pb2 + 2 KI ? PbI2 + 2 KNO3
Solution: Do two stoichiometry calculations of the same sort we learned earlier. The first stoichiometry calculation will be performed using “1.75 mol O2” as our starting point, and the second will be performed using “2.75 mol H2” as our starting point.
- 1.75 mol O22 mol H2O?1 molO2 = 3.50 mol H2O
- 2.75 mol H22 mol H2O?2 mol H2 = 2.75 mol H2O
Because “2.75 mol O2” is the smaller of these two answers, it is the amount of water that we can actually make. The limiting reactant is hydrogen because it is the reactant that limits the amount of water that can be formed since there is less of it than oxygen.
What Is The Benefit Of Having A Limiting Reagent
In a chemical reaction, the task of limiting reagent or reactant is significant because it can help the chemist predict the maximum quantity of reactant is consumed, since it restricts the reaction, only the necessary moles of products can be produced instead of the hypothetical yield where the perfect quantity is used.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
How To Identify Limiting Reactant
To identify the limiting reactant in a chemical reaction, the mole number of reactant and product formed after completion of a reaction should be calculated. With that, the mole numbers of unreacted species should also be required.
The limiting reactant can also be identified by comparing the quantity of products formed from each of the reactant. The reagent produces lesser number of product with comparing to the other reactant is decided as limiting reagent of that chemical reaction.
The reactant which is present in relatively smaller amount than the required amount by stoichiometry will be the limiting agent.
It can be explained through the following example-
Question:Which one will be the limiting and excess reactant when 4g of hydrogen takes part in a reaction with 48 g of Oxygen?
Read Also: How To Create Chemistry Between Characters
What Is Limiting Reactant
Based on their amounts and their roles, we classify reactants into two kinds: limiting reactants and excess reactants.
Limiting reactants are those that get completely utilized in a reaction first and thus limit the amount of product that will be produced. Excess reactants, on the other hand, are the reactants that are still present after the reaction has reached a standstill.
Lets say that youre standing in a queue at your favorite bagel vendor. The bagel guy comes out of the cart and announces that he only has 10 bagels left. Now, you look up from your phone and start counting the number of people ahead of you in the queue. You count a total of 20 people, which is 10 more than the number of bagels that remain.
In our example, the customers standing in the queue and the bagels are reactants, coming together to produce happy-fed individuals .
However, the number of bagels will limit the number of happy-fed customers that can be achieved in the end, making it the limiting factor , while the customers will be considered the excess reactants.
How To Find Limiting Reagent
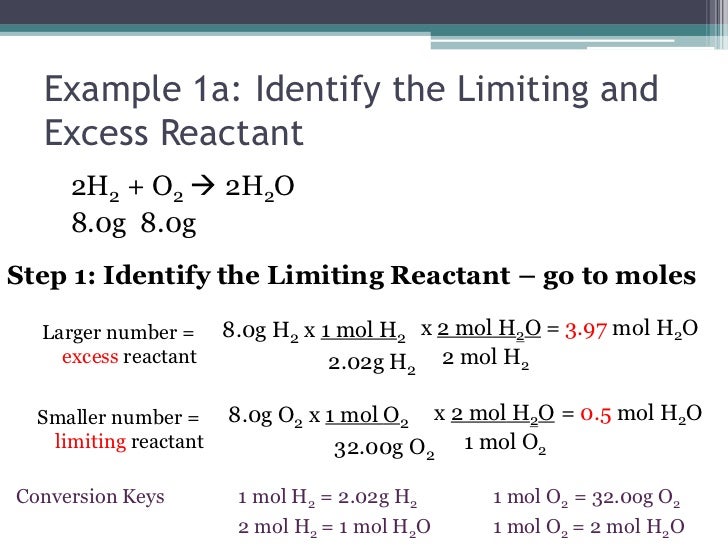
The determination of the limiting reactant is typically just a piece of a larger puzzle. In most limiting reactant stoichiometry problems, the real goal is to determine how much product could be formed from a particular reactant mixture. The limiting reactant or reagent can be determined by two methods.
In order to calculate the mass of the product first, write the balanced equation and find out which reagent is in excess. Using the limiting reagent calculate the mass of the product.
The following points should be considered while attempting to identify the limiting reagent:
- When there are only two reactants, write the balanced chemical equation and check the amount of reactant B required to react with reactant A. When the amount of reactant B is greater, the reactant A is the limiting reagent.
- The reactant which is in a lesser amount than is required by stoichiometry is the limiting reactant.
- In an alternate method of finding the limiting agent, the amount of product formed by each reactant is calculated.
- The limiting reactant is the reactant from which the minimum amount of product is formed.
- Also, if we calculate the amount of one reactant needed to react with another reactant, then the reactant which is in shortage would be the required limiting reactant.
You May Like: What Influence Did Geography Have On The Development Of Greek Society
Comparison Of Product Amounts Which Can Be Formed From Each Reactant
In this method the chemical equation is used to calculate the amount of one product which can be formed from each reactant in the amount present. The limiting reactant is the one which can form the smallest amount of the product considered. This method can be extended to any number of reactants more easily than the first method.
What Is A Limiting Reagent
The limiting reactant is the reagent to be totally consumed in a chemical reaction. Limiting reactant is also what prevents a reaction from continuing because there is none left. The limiting reactant may also be referred to as limiting reagent or limiting agent. The reactant that is not used up is referred to as the excess reactant.
For example, consider if you are trying to put together a burger that has two pieces of bread, a piece of tomato, and a piece of meat. The reaction would be
1 Tomato + 1 Meat + 2 Bread > 1 Burger
You have 3 tomato pieces, 2 pieces of meat, and 6 pieces of bread.
When combined to make sandwiches, the first ingredient to run out is the meat. You only have enough meat to make two burgers but enough tomato and bread to make three burgers Therefore, the meat is the limiting reagent. The meat is the first ingredient to be used up. The bread and tomato have excess and therefore are called the excess reactant. The same idea is true for chemical reactions.
It is important to be able to find the limiting reagent because we cant always add exactly the number of molecules we want to perfectly balance our equations. Often time, one reagent will be added in excess because it is cheaper and make sure the more expensive reagent is completely used up.
Also Check: Geometry Escape Challenge A Answer Key
Example Question #: Identifying Limiting Reagents
Consider the following reaction:
If the reaction starts initially with of nitrogen gas and
Both will run out at the same time
Correct answer:
Nitrogen gas
In order to determine the limiting reactant, we can use a calculation to determine how much of one reactant is needed in order to use up the other. For example, we can see how much hydrogen gas is necessary in order to use up all of nitrogen gas that we have:
In other words, we need of hydrogen gas in order to use up of nitrogen gas. Since we have of hydrogen gas, we have more than enough to react all of the nitrogen gas, and the nitrogen gas will be used up before the hydrogen gas. As a result, nitrogen gas is the limiting reactant.
Gram ratio of A to B
Pressure ratio of A to B
Molar ratio of A to B
Volume ratio of A to B
Correct answer:
Molar ratio of A to B
Limiting reactants are determined when there is an excess of a particular reactant, in relation to the other reactants available. When reactants are compared, one must always compare the molar ratio in order to determine which reactant is limited. In this reaction, if we are given the available amount of each reactant, we will need to convert the given amount of one reactant to the necessary amount of the other required to fully react. If we find that reactant A is available in excess, then reactant B will be the limiting reagent. The only way to compare these two terms, however, is by using the molar ratio in the reaction.
2g of hydrogen gas and 16g of oxygen gas
How To Find The Limiting Reactant
There are a couple of ways by which one can determine the limiting and excess reactants in a reaction. However, the methods come with a prerequisite, which is to have a balanced chemical equation in hand.
The methods use stoichiometric coefficients from the balanced chemical equation to calculate ratios if the coefficients themselves are incorrect, the final answers obtained will also be incorrect.
In the first method, we will find and compare the mole ratios of the reactants, while in the other one, we will find the amount of product that will be produced by each reactant. The one that produces the least amount of the end product is the limiting reagent.
Read Also: Paris Katherine Jackson Biological Father
How To Find Excess Reagent In Moles
The following procedures should be followed to determine the excess reactant and the quantity of excess reactant in a chemical reaction.
Balanced equation consisting of all reactant and product should be formed. The mole number of product formed will be calculated from each of the reactant present of the chemical reaction respectively.The reactant forming larger amount of product is the excess reagent.Now the mass of the excess reactant that is consumed in the reaction and the mass that is unreacted should be measured respectively.
This is explained through the following example.
Question: Which one is the excess reactant when 80 g of Na2O2 combines with 30 g of water?
What Is Limiting Reagents
The reactant that is entirely used up in a reaction is called as limiting reagent.
Limiting reagents are the substances that are completely consumed in the completion of a chemical reaction. They are also referred to as limiting agents or limiting reactants. According to the stoichiometry of chemical reactions, a fixed amount of reactants is required for the completion of the reaction. Let us consider the following reaction of formation of ammonia:
3H2 + N2 2NH3
In the reaction given above, 3 moles of Hydrogen gas are required to react with 1 mole of nitrogen gas to form 2 moles of ammonia. But what if, during the reaction, only 2 moles of hydrogen gas are available along with 1 mole of nitrogen.
In that case, the entire quantity of nitrogen cannot be used . Hence, the hydrogen gas is limiting the reaction and is therefore called the limiting reagent for this reaction.
Also Check: Paris Jackson’s Father
How To Find Limiting Reactant Without Mass
Firstly, the balanced equation of that particular reaction should be developed.
Then the mass of the individual reactant before the reaction should be noted and the leftover mass of each of the reactant should be measured.
Those reactant remains excess and unreacted after the reaction are the excess reagent and those are completely used up are limiting reagent.
To know more please follow:SN2 Examples: Detailed Insights And Facts
How To Calculate Moles Of A Reactant
Calculating moles of any compound is very important part of any chemical reaction to determine how much reactant is reacted and how much product will be formed. Mole number also helps to find the concentration of any particular solution.
At first molar atomic mass of that compound should be known to calculate the mole number. Then the mass of reactant should be provided. Mole number can be detected by dividing the mass of the reactant with the molar atomic mass of the reactant.
No of moles =
This is explained through an example below-
Question: How many moles of Glucose is present in 60g of Glucose?
Molar atomic weight of Glucose is 180.06 gm.mol-1
Given mass of glucose = 60g
No of moles of Glucose =
= 0.333
You May Like: Ccl4 Molecular Structure
What Is Limiting Reagent
Limiting reagent in a reaction is the first to be fully used up and thus stops any further reaction from occurring.
In a given chemical reaction, the limiting reagent, or it is also known as limiting reactant, is the substance that has been fully consumed when the chemical reaction is completed.
With the help of stoichiometry, the exact amount of reactant which is needed to react with another element can be calculated.
Though, if the reagents are not mixed or are present in these precise stoichiometric proportions, then the limiting reagent will be fully consumed and the given reaction will not go to stoichiometric completion.
Thus, in simple terms, the reactant that is fully used up in a reaction is called a limiting reagent.
This reactant called limiting reagent generally governs when the reaction will stop.
The exact amount of reactant which will be required to react with another element can be calculated from the reaction stoichiometry.
The limiting reagent depends only on the mole ratio, not on the masses of the reactants present in the given chemical equation.
Mass Relationships And Chemical Equations
- Determine the limiting reagent and the amount of a product formed in a given reavion
Key Points
- The limiting reagent is the reactant that is used up completely. This stops the reaction and no further products are made.
- Given the balanced chemical equation that describes the reaction, there are several ways to identify the limiting reagent.
- One way to determine the limiting reagent is to compare the mole ratios of the amounts of reactants used. This method is most useful when there are only two reactants.
- The limiting reagent can also be derived by comparing the amount of products that can be formed from each reactant.
Term
- limiting reagentThe reactant in a chemical reaction that is consumed first prevents any further reaction from occurring.
In a chemical reaction, the limiting reagent, or limiting reactant, is the substance that has been completely consumed when the chemical reaction is complete. The amount of product produced by the reaction is limited by this reactant because the reaction cannot proceed further without it often, other reagents are present in excess of the quantities required to to react with the limiting reagent. From stoichiometry, the exact amount of reactant needed to react with another element can be calculated. However, if the reagents are not mixed or present in these correct stoichiometric proportions, the limiting reagent will be entirely consumed and the reaction will not go to stoichiometric completion.
Limiting reagent
Recommended Reading: Segment And Angle Addition Worksheet Answers
How To Find The Limiting Reactant In A Chemical Reaction
The limiting reagent in a reaction is found by calculating the amount of product produced by each reactant. The reactant that produces the least amount of product is the limiting reactant.
There are many things that need to go right for a chemical reaction to yield useful products: from the environment surrounding the reaction to the amount of the reactants present. Only once in a blue moon do all the reactants get converted into products.
In most reactions, one reagent is entirely depleted, while some quantity of the other reagents stays available for further reaction.
Since one of the reactants is not always available, the reaction hits a roadblock and does not continue. This reactant that gets completely used up, and thus limits the reaction from advancing forward, is called the limiting reactant or limiting reagent.
The limiting reagent in a chemical reaction controls how much of the final product will be produced.
Recommended Video for you:
How To Find Limiting Reagent In A Reaction
Let us now learn about how to determine limiting reagent in a reaction.
There are two ways for how to calculate limiting reagent. One method is to find and compare the mole ratio of the reactants that are used in the reaction. Another method is to calculate the grams of products produced from the quantities of reactants in which the reactant which produces the smallest amount of product is the limiting reagent.
Method 1: Finding the limiting reagent by looking at the number of moles of every reactant.
First, determine the balanced chemical equation for the given chemical reaction.
Then, convert all the given information into moles .
The next step is to calculate the mole ratio from the given information. Then, compare the calculated ratio to the actual ratio.
Use the amount of limiting reactant for calculating the amount of product produced.
Lastly, if necessary, calculate how much of the non-limiting agent is left in excess.
Method 2: Finding the limiting reagent by calculating and comparing the amount of product each reactant would produce.
The first step is to balance the chemical equation for the given chemical reaction.
Then, convert the given information into moles.
Use stoichiometry for each individual reactant for finding the mass of product produced.
The reactant which produces a lesser amount of product would be the limiting reagent.
The reactant which produces a larger amount of product would be the excess reagent.
Don’t Miss: Negative Work Physics
Is It Possible To Calculate The Percentage Of Yield From Limiting Reactant
Answer: Yes, the percentage of yield can be calculated from the concept of limiting reactant.
An example is shown below-
If 25 ml of 0.320 M barium chloride takes part in a reaction with excess amount of silver nitrate and form the silver chloride precipitate. 1.83 g of silver chloride is collected as precipitate. What will be the percentage of yield of this reaction?
BaCl2 + 2AgNO3 = 2AgCl + Ba2
Mole number of BaCl2 = 0.320 molar BaCl2
=8 ×10-3
Theoretical yield of AgCl precipitate=8 ×10-3 mol BaCl2 × ×
=2.2928 g AgCl
% yield = ×100%
= ×100%