Calculating The Volume Of A Cylinder
How To Calculate Mass Percent Concentration Of A Solution
Mass percent composition is the easiest way to express the concentration of a solution because no unit conversions are required. Simply use a scale to measure the mass of the solute and the final solution and express the ratio as a percentage. Remember, the sum of all percentages of components in a solution must add up to 100%
Mass percent is used for all sorts of solutions but is particularly useful when dealing with mixtures of solids or anytime physical properties of the solution are more important than chemical properties.
Calculate Mass Percent: mass solute divided by mass final solution multiplied by 100%
symbol: %
Example: The alloy Nichrome consists of 75% nickel, 12% iron, 11% chromium, 2% manganese, by mass. If you have 250 grams of nichrome, how much iron do you have?
Because the concentration is a percent, you know a 100-gram sample would contain 12 grams of iron. You can set this up as an equation and solve for the unknown “x”:
12 g iron / 100 g sample = x g iron / 250 g sample
Cross-multiply and divide:
x= / 100 = 30 grams of iron
Show Students Five Rods That Have The Same Mass But Different Volumes
Show students the five rods and explain that they all have the same mass. Then hold up the longest, middle-sized, and shortest rods and remind students that they have the same mass.
Ask students to make a prediction:
Students may reason that since the mass of each rod is the same, the volume of each rod must have something to do with its density. Some may go so far as to say that the rod with the smallest volume must have the highest density, because the same mass is packed into the smallest volume. Or that the rod with the largest volume must have the lowest density, because the same mass is spread out over the largest volume.
Tell students that like the cubes in the previous activity, they will need to know the volume and mass of each of the samples. They will also calculate the density of each sample and use this value to figure out which material each rod is made of.
Don’t Miss: Elton John Kids Adopted
Calculating The Volume Of A Rectangular Prism
How To Calculate Molality Of A Solution
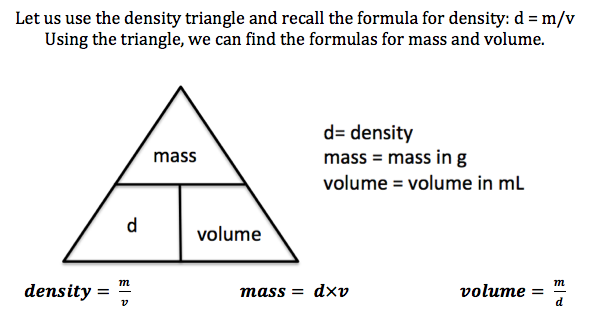
Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same.
Calculate Molality: moles solute per kilogram solvent
symbol: m
m = moles / kilogram
Example: What is the molality of a solution of 3 grams of KCl in 250 ml of water?
First, determine how many moles are present in 3 grams of KCl. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. Then add them together to get the grams per mole for KCl.
- K = 39.1 g/mol
- KCl = 39.1 + 35.5 = 74.6 g/mol
For 3 grams of KCl, the number of moles is:
* 3 grams = 3 / 74.6 = 0.040 moles
Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water , but you also have 3 grams of solute, so the total mass of the solution is closer to 253 grams than 250. Using 2 significant figures, it’s the same thing. If you have more precise measurements, don’t forget to include the mass of solute in your calculation!
- 250 g = 0.25 kg
- m = 0.040 moles / 0.25 kg = 0.16 m KCl
Also Check: Movement In Geography Examples
How To Calculate Molarity Of A Chemical Solution
Molarity is one of the most common units of concentration. It is used when the temperature of an experiment won’t change. It’s one of the easiest units to calculate.
Calculate Molarity: moles solute per liter of solution
symbol: M
M = moles / liter
Example: What is the molarity of a solution of 6 grams of NaCl dissolved in 500 milliliters of water?
First, convert grams of NaCl to moles of NaCl.
From the periodic table:
- NaCl = 23.0 g/mol + 35.5 g/mol = 58.5 g/mol
- Total number of moles = * 6 g = 0.62 moles
Now determine moles per liter of solution:
M = 0.62 moles NaCl / 0.50 liter solution = 1.2 M solution
Note that I assumed dissolving the 6 grams of salt did not appreciably affect the volume of the solution. When you prepare a molar solution, avoid this problem by adding solvent to your solute to reach a specific volume.
Calculating The Volume Of A Sphere
Read Also: Michael Jacksons Biological Son
Key Concepts And Summary
The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change . The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure . The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant . Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules .
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal gas constant.
How To Find The Formula For Specific Volume
Specific volume is then calculated as volume divided by mass. Notice that since density is mass over volume, specific volume can also be defined as the inverse of density. So you can also calculate specific volume by using the formula for inverse density: To better imagine this, let’s say you have a container with a certain amount of air inside.
Also Check: Different Types Of Lines In Geometry
How To Calculate Normality Of A Chemical Solution
Normality is similar to molarity, except it expresses the number of active grams of a solute per liter of solution. This is the gram equivalent weight of solute per liter of solution.
Normality is often used in acid-base reactions or when dealing with acids or bases.
Calculate Normality: grams active solute per liter of solution
symbol: N
Example: For acid-base reactions, what would be the normality of 1 M solution of sulfuric acid in water?
Sulfuric acid is a strong acid that completely dissociates into its ions, H+ and SO42-, in aqueous solution. You know there are 2 moles of H+ ions for every 1 mole of sulfuric acid because of the subscript in the chemical formula. So, a 1 M solution of sulfuric acid would be a 2 N solution.
How To Find Volume Chemistry Calculator
Find the volume of the cylinder using the formula r²h. Find the surface area of the cylinder using the formula 2rh + 2r2. Make a ratio out of the two formulas, i.e. r 2 h : 2rh + 2r 2. Alternatively, simplify it to rh : 2 . Divide both sides by one of the sides to get the ratio in its simplest form.
Don’t Miss: Jonathan Tennant Beth Thomas
Breathing And Boyles Law
What do you do about 20 times per minute for your whole life, without break, and often without even being aware of it? The answer, of course, is respiration, or breathing. How does it work? It turns out that the gas laws apply here. Your lungs take in gas that your body needs and get rid of waste gas . Lungs are made of spongy, stretchy tissue that expands and contracts while you breathe. When you inhale, your diaphragm and intercostal muscles contract, expanding your chest cavity and making your lung volume larger. The increase in volume leads to a decrease in pressure . This causes air to flow into the lungs . When you exhale, the process reverses: Your diaphragm and rib muscles relax, your chest cavity contracts, and your lung volume decreases, causing the pressure to increase , and air flows out of the lungs . You then breathe in and out again, and again, repeating this Boyles law cycle for the rest of your life .
Figure 7.
Finding Concentration In Percentage Or Parts Per Million

You May Like: Geometry Dash 1 20
How Can I Estimate The Volume Of A Molecule
- I would like to know the size of one molecule of methyl orange? Udit Saxena 3/16/99
-
If the volume of empty space in a solid is negligible compared to the volume occupied by molecules,a quick and dirty estimate of the molecular volume can be obtained by dividing the volume of one mole of the material by Avogadro’s number. To compute the molar volume, you need the material’s density and its molecular weight. For methyl orange, the density of the solid is approximately 1.00 g/mL,and the molecular weight is 327.34, so the molar volume is
× = 327 mL/mol
327 mL/mol & times = 5.43 & times 10-22 mL/molecule
These aren’t really actual molecular volumes they’re the average contribution each molecule makes to the total volume of the crystal. When the molecules are packed in a regular way , these average molecular volumes are easy to relate to the average center-to-center distance between molecules. The relationships are sometimes used to explain volume changes that accompany phase changes.
How To Use Our Volume To Mass Calculator
First, you need density. Check if the material is on our list or input known density.
Second, input volume or mass in the correct unit. Change the unit first before you enter a number.
That’s it! The volume to mass calculator will find the result in less than a second!
Here are the products and their densities available in our calculator:
- Food:
- Walnuts, hazelnuts, grounded – 520 kg/m3
- Sesame – 640 kg/m3
- Cream 38% fat – 984 kg/m3
- Cream 13% fat – 1,013 kg/m3
- Air – 1.205 kg/m3
- Carbon dioxide – 1.977 kg/m3
- Carbon dioxide – 1.842 kg/m3
- Carbon monoxide – 1.250 kg/m3
- Carbon monoxide – 1.165 kg/m3
- Hydrogen – 0.0898 kg/m3
- Methane – 0.717 kg/m3
- Methane – 0.688 kg/m3
- Nitrogen – 1.2506 kg/m3
- Nitrogen – 1.165 kg/m3
- Oxygen – 1.4290 kg/m3
- Oxygen – 1.331 kg/m3
- Propane – 1.882 kg/m3
- Water vapor – 0.804 kg/m3
- Liquid hydrogen – 70 kg/m3
- Liquid oxygen – 1,141 kg/m3
- Water – 1,000 kg/m3
- Water – 1,030 kg/m3
- Astronomy:
- The Universe – 5·10-27 kg/m3
- Interstellar medium – 1*10-19 kg/m3
- Earth’s inner core – 13,000 kg/m3
- Sun’s core – 33,000 kg/m3
- Sun’s core – 160,000 kg/m3
Also Check: Paris Jackson Parents
Chemistry End Of Chapter Exercises
the appropriate graph
Boyles law