What Is Green Chemistry
The concept of greening chemistry developed in the business and regulatory communities as a natural evolution of pollution prevention initiatives. In our efforts to improve crop protection, commercial products and medicines, we also caused unintended harm to our planet and humans.
Green chemistry takes the EPA’s mandate a step further and creates a new reality for chemistry and engineering by asking chemists and engineers to design chemicals, chemical processes and commercial products in a way that, at the very least, avoids the creation of toxics and waste.
Green Chemistry is not politics. Green Chemistry is not a public relations ploy. Green chemistry is not a pipe dream.
We are able to develop chemical processes and earth-friendly products that will prevent pollution in the first place. Through the practice of green chemistry, we can create alternatives to hazardous substances. We can design chemical processes that reduce waste and reduce demand on diminishing resources. We can employ processes that use smaller amounts of energy. We can do all of this and still maintain economic growth and opportunities while providing affordable products and services to a growing world population.
This is a field open for innovation, new ideas, and revolutionary progress. This is the future of chemistry. This is green chemistry.
Getting Rid Of Dangerous Heavy Metals
Its not just solvents that chemists are trying to eliminate from manufacturing processes there are many other chemicals used in manufacture which can be damaging to the environment. For example, scientists at Pfizer faced a major challenge in improving the manufacture of sildenafil citrate1, the active ingredient in ViagraTM and RevatioTM, a drug used to treat pulmonary hypertension.
The traditional process for manufacturing sildenafil citrate relied on a tin chloride catalyst, as well as chlorinated and volatile solvents. Tin is a heavy metal and a major environmental polluter. Chemists cleaned up the process by replacing the tin reaction with a catalytic hydrogenation. With only water as a by-product, catalytic hydrogenation is one of the cleanest possible chemical reaction steps.
But chemists went even further than this, and eventually created an entirely new synthetic route to make sildenafil citrate, built around a very efficient final step. This new route is predicted to have eliminated 30,000 tonnes of chemical waste between 1997 and 2013.
Advances in Green chemistry will continue to offer opportunities to discover and apply new chemistry, to improve the economics of chemical manufacturing and reduce the chemical related environmental impact.
Carbon Dioxide As Blowing Agent
In 1996, Dow Chemical won the 1996 Greener Reaction Conditions award for their 100% carbon dioxide blowing agent for polystyrene foam production. Polystyrene foam is a common material used in packing and food transportation. Seven hundred million pounds are produced each year in the United States alone. Traditionally, CFC and other ozone-depleting chemicals were used in the production process of the foam sheets, presenting a serious environmental hazard. Flammable, explosive, and, in some cases toxic hydrocarbons have also been used as CFC replacements, but they present their own problems. Dow Chemical discovered that supercritical carbon dioxide works equally as well as a blowing agent, without the need for hazardous substances, allowing the polystyrene to be more easily recycled. The CO2 used in the process is reused from other industries, so the net carbon released from the process is zero.
Recommended Reading: Who Is Paris Jackson’s Biological Father
Less Hazardous Chemical Synthesis
The family of polycarbonates contains very important polymers which are used where high optical properties combined with strength are needed. The polycarbonate most used is manufactured from bisphenol A, whose structure is:
The polycarbonate is manufactured by a condensation reaction between bisphenol A and either carbonyl chloride or diphenyl carbonate.
Carbonyl chloride is a very poisonous gas, manufactured from other hazardous gases, carbon monoxide and chlorine:
On the other hand, diphenyl carbonate is produced from dimethyl carbonate, which is readily manufactured from methanol, carbon monoxide and oxygen in the liquid phase, in presence of copper chloride, CuCl2:
Dimethyl carbonate, when heated with phenol in the liquid phase, forms the diphenyl carbonate:
Overall, the process for the production of polycarbonate that uses diphenyl carbonate is less hazardous than that using carbonyl chloride.
Green Chemistry Is An Alternative Tool For Reducing Pollution:
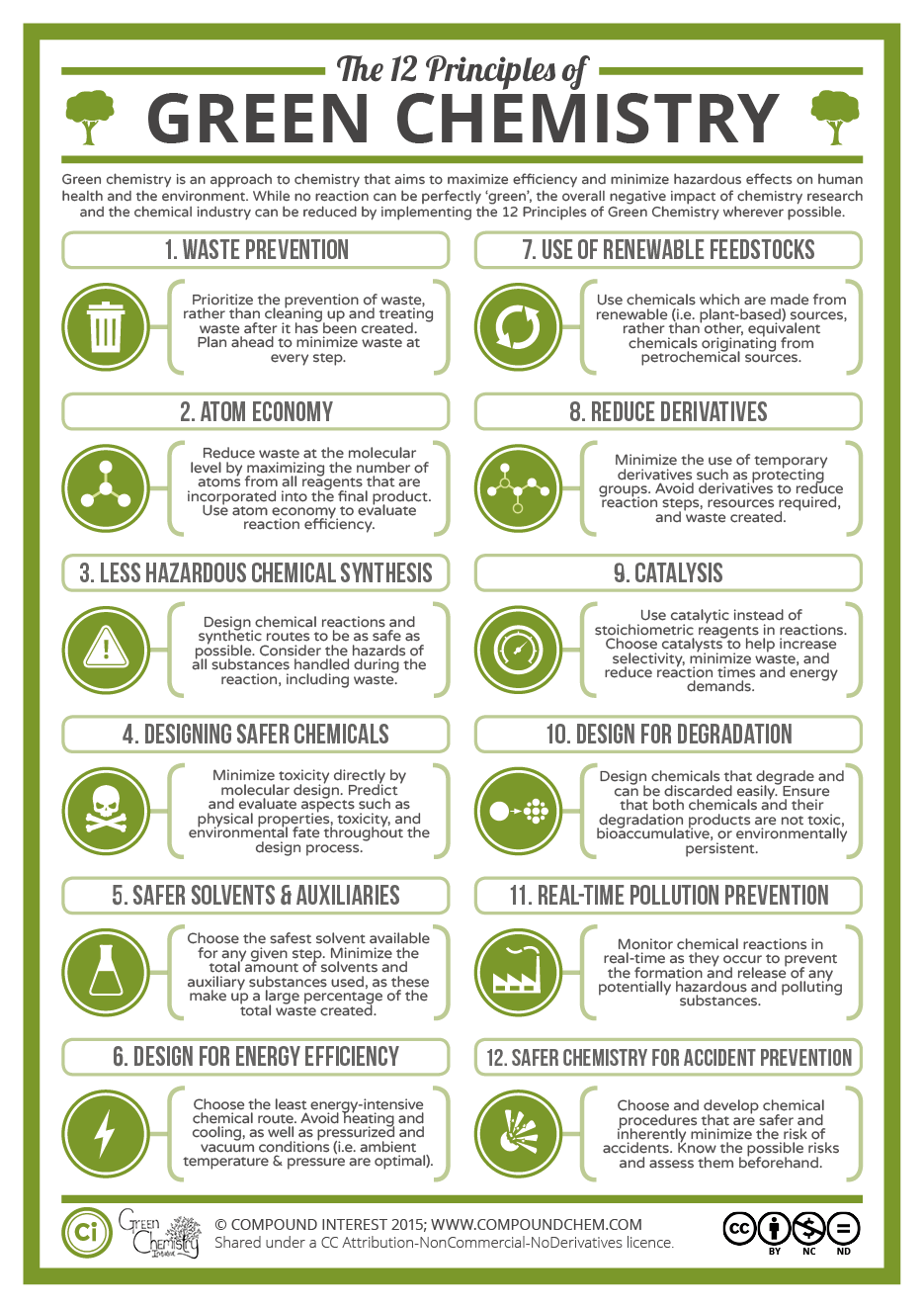
Twelve Principles of Green Chemistry-This is true that green chemistry is an alternative tool for reducing pollution. The following principles justify the same:
Prevention: Preventing waste is better than cleaning up debris. It helps inefficient use of resources and prevents waste.
Atom Economy: Innovation of synthetic methods to maximize the incorporation of all materials used in the process into the final product. It will result in less generation of waste.
Less Hazardous Chemical Syntheses: Synthetic techniques should avoid using or producing toxic substances to human health or the environment.
Designing Safer Chemicals: Chemical products should be made to achieve their desired function while being as non-toxic as possible. Minimizing toxicity, while simultaneously maintaining function and efficacy, maybe one of the most challenging things in designing safer products and processes. Achieving this goal requires an understanding of not only chemistry but also the principles of toxicology and environmental science. Chemists often used these highly reactive chemicals to manufacture products. Therefore, complete knowledge with excellent skills is required to design safer chemicals.
Safer Solvents and Auxiliaries: Auxiliary substances should be avoided wherever possible, and as non-hazardous as possible when they must be used.
Read Also: Angle Addition Worksheets
How Does Green Chemistry Save The Environment
As shown above, green chemistry saves the environment in several ways. These 12 principles can be classified into two main categories reducing risk and minimising the environmental impact.
For example, designing chemicals and manufacturing processes that have low or zero waste byproducts can help to prevent possible toxic effects and contamination. However, its not only about sustainability cost-effectiveness is an important consideration too.
Energy Efficiency And Use Of Waste Materials
All manufacturing processes need energy to convert raw materials into useful products. In the chemical industry it is used to heat reactants and in processes such as distillation, product drying, electrolysis, and treatment of waste.
At present, the energy used still relies mainly on fossil fuels, but even so the use of these can be reduced in several ways .
| Maintenance and recovery | Good insulation and well-maintained equipment will reduce heat loss, and any waste heat can be used for warming offices and producing hot water rather than being lost to the atmosphere. In some cases this heat may be shared with a local community by piping hot water from the site. |
| Reaction choice and conditions | Reactions and catalysts that operate at lower temperatures may be chosen.Most reactions based on biosynthesis work at relatively low temperatures however this may need balancing with the extra energy often needed for product separation. |
| Combined heat and power | Manufacturing sites often generate their own electricity, rather than buying from the grid. This is more efficient as it eliminates transmission losses, and the excess heat released during the generation process can be used on site for many different purposes from pre-heating reactants to keeping offices warm. |
Table 2 Improving energy efficiency in the chemical industry.
Don’t Miss: What Is Vo In Physics
Frequently Asked Questions On Green Chemistry
Q.1: Who invented Green Chemistry?Ans.The idea of green chemistry was developed initially as a reaction to the Pollution Prevention Act of \, in the U.S. to eliminate pollution by the improved the design of chemicals used in various fields. At that time Paul Anastas and John C. Warner invented the idea of green chemistry.
Q.2: What are the 12 principles of Green Chemistry?Ans: The twelve principles of green chemistry are: Prevention, Atom economy, Less hazardous chemical syntheses, Designing safer chemicals, Safer solvents, and auxiliaries, Design for energy efficiency, Use of renewable feedstock, Reduce derivatives, Catalysis, Design for degradation, Real-time analysis for pollution prevention, Inherently safer chemistry for accident prevention.
Q.3: Where is Green Chemistry used?Ans.Green chemistry is used in the chemical industry it is used in processes such as distillation, product drying, electrolysis, and waste treatment. Presently, the energy used in chemical industries are still relying mainly on fossil fuels, but still, the use of these non-renewable sources of energy can be reduced in several ways.
Q.4: What is Green Chemistry?Ans. Green Chemistry, also known as sustainable chemistry is the design of chemical products and processes that eliminate or minimize the use or generation of hazardous chemical substances. It prevents pollution at the initial molecular level.
Safer Solvents And Auxiliaries
The use of auxiliary substances should be made unnecessary wherever possible and, innocuous when used.
Dr. Concepcíon Jiménez-González, Director, Operational Sustainability, GlaxoSmithKline
It was a green chemistry conference and the very famous synthetic chemist had just received a question about why he had chosen a solvent that was without question a very poor choice. You have to be realistic, chemists know intuitively what’s best, and solvents don’t matter. It’s the chemistry that counts. I’ve heard this kind of remark repeatedly over many years, despite the fact that it goes against the spirit and letter of Principle 5.
Solvents and mass separation agents of all kinds matter a lot to the chemistry not to mention the chemical process and the overall “greenness” of the reaction. In many cases, reactions wouldn’t proceed without solvents and/or mass separation agents. To say that they don’t matter, or that it’s only the chemistry that counts is not just a logical fallacy, it’s chemically incorrect. Solvents and separation agents provide for mass and energy transfer and without this, many reactions will not proceed.
It has also been shown that solvents account for 50 80 percent of the mass in a standard batch chemical operation, depending on whether you include water or you don’t. Moreover, solvents account for about 75% of the cumulative life cycle environmental impacts of a standard batch chemical operation.
Read Also: What Does Ml Stand For In Chemistry
It Is Better To Prevent Waste Than To Treat Or Clean Up Waste After Its Formation
This statement is one of the most popular guidelines in process optimization it describes the ability of chemists to redesign chemical transformations in order to minimize the generation of hazardous waste as an important step toward pollution prevention. By preventing waste generation, the hazards associated with waste storage, transportation, and treatment could be minimized.
This principle is easy to understand and easy to apply, and examples from both industry and academia have proven its significance, relevance, and feasibility. This pillar of green chemistry is still valid however, we have to conceive it in a broader context, switching from a restricted interpretation of waste based on its quantity to a universal approach to deal with the topic waste: We have to take wastes multidimensional nature into account. We need to move from a quantity of waste per quantity of product principle toward a principle addressing the quantity of waste generated per function provided by the product. In this context, we have to aim at making both quality and functionality of products superior. Considering the entire life cycle of a product, we have to address the fact that not only the production process itself generates waste but, moreover, end-of-life waste accrues after the products life span or its consumption. This encompasses firstly the conversion of such materials up to now considered as waste into valuable products and, secondly, their recyclability.
The Concept Of Atom Economy
When chemists are considering a compound, they are concerned with the chemical, biological, and physical properties of this compound, and the
method by which the compound is prepared or its synthesis . In order to focus greater attention on waste by-products that are formed during a synthesis, Barry Trost of Stanford University developed the concept of atom economy. This concept deals with the question: How many of the atoms of the reactants are incorporated into the final desired product and how many are wasted by incorporation into by-products? An example of the application of this concept is discussed in the following synthesis of ibuprofen.
Pesticides. Dichlorodiphenyl trichloroethane is one of the most well-known insecticides. During World War II it saved thousands of Allied lives by killing disease-carrying insects, but during the 1960s the significant environmental damage caused by DDT was brought to the public’s attention by Rachel Carson in Silent Spring . As a result of the controversy generated by this book and other media coverage, the substance’s use was banned in the United States in 1973.
During the 1960s and 1970s organophosphates largely replaced organo-chlorine pesticides such as DDT. These pesticides rapidly degrade in the environment, but they are much more toxic to mammals. They are deadly to a wide array of insects and kill not only the target organism but also beneficial insects, such as bees and predatory beetles, and can also be harmful to humans.
You May Like: Imagine Math Parent Portal
Safer Solvent And Auxiliaries
Most of the industries from polymer to pharmaceutical industries and other chemical allied industries use solvents at some point in their manufacturing. In general, the use of solvents should be avoided, but that is not possible in all cases. So we can possibly replace toxic, non-recyclable solvents with safer and innocuous solvents.
As a case study, a good success story in such a replacement is Bayer in 2000, effectively replacing Volatile Organic Compounds in their polyurethane coatings with water. While in another case, even cost-intensive Ionic Liquids are successfully used as solvent in Alkylation of isobutene by China Petroleum and Chemical Corp. in 2019. They produce 300,000 tonnes/year of high-quality alkylate is one of the recent adoptions of Ionic Liquids in the industrial scale.
Image courtesy: VectorStock®
Benefits Of Green Chemistry
Human health:
- Cleaner air: Less release of hazardous chemicals to air leading to less damage to lungs
- Cleaner water: less release of hazardous chemical wastes to water leading to cleaner drinking and recreational water
- Increased safety for workers in the chemical industry less use of toxic materials less personal protective equipment required less potential for accidents
- Safer consumer products of all types: new, safer products will become available for purchase some products will be made with less waste some products will be replacements for less safe products
- Safer food: elimination of persistent toxic chemicals that can enter the food chain safer pesticides that are toxic only to specific pests and degrade rapidly after use
- Less exposure to such toxic chemicals as endocrine disruptors
Environment:
- Many chemicals end up in the environment by intentional release during use , by unintended releases , or by disposal. Green chemicals either degrade to innocuous products or are recovered for further use
- Plants and animals suffer less harm from toxic chemicals in the environment
- Lower potential for global warming, ozone depletion, and smog formation
- Less chemical disruption of ecosystems
- Less use of landfills, especially hazardous waste landfills
Economy and business:
Read Also: Prince Jackson Real Father
What Are The Costs That Go Into Our Laboratories
From our position as facilitators: chemicals, waste disposal, materials , staff ), and overhead for equipment/systems, time. It is always a matter of priority, the question of where does it make sense to try to save money? We are clear that we do not want to compromise our academic goals of providing students with a strong foundation of chemical principles, but we are finding that changing some of our current experiments and practices may even enhance our programme.
Even though equipment such as analytical instruments represents a significant expense, they are hassle free for several years due to warranty and the fact that they should last long enough before we may find the need to replace a substantial number of units. We will focus on other expenses and how they are associated with turning into green practices.
The easiest expense to cut down is the purchase of chemicals. If we decide to remove an experiment that uses an expensive chemical and replace it with a cheaper alternative, there are savings associated with the lower cost of the purchase and also the need or not of disposal of the post-experiment excess and by-products.
Our goal is to be able to provide similar alternatives to at least 50% of our general chemistry experiments within the next 5 years.
Impact Of Green Chemistry
Green Chemistry is a proactive approach to pollution Prevention.
Green Chemistry is based on principles like
-
Waste minimization at source
-
Use of catalyst in place regents
-
Using non-toxic reagents
-
Use of solvent-free or recyclables
-
Environmental benign solvent system.
Green chemistry-topic in chemical engineering.
Grew chemistry studies about the alternatives of toxic solvents which are renewable in nature.
Thus, green chemistry has great potential in reducing the toxicity of industrial domains by developing safer options.
Know more about solvent and its examples by clicking here solvent.
Read Also: Find The Message Pre Algebra With Pizzazz
Goals Of Green Chemistry
The principles and guidelines of Green Chemistry are intended to fulfill the following goals for any chemical process, whether industrial or laboratory scale:
-
Make better use of available resources for the development of a chemical process.
-
Reduce waste generated in any preparation or handling of chemicals.
-
Materials should be prepared by improved processes that reduce unwanted effects on the environment.
-
Replace toxic reagents and products with others that have the same properties and applications but have less impact on the environment.
-
Reduce the energy required to produce substances of interest, either by the use of much faster processes or by the use of renewable energies involving lower energy cost with equal efficiency.
-
Reduce toxicity or general danger for a given compound substance and the compound itself.
-
Reduce costs by eliminating any manipulation that is not strictly necessary and decreasing time invested in the preparation of a substance.
-
Analyzing Waste Streams 54
- II.B.
-
Atom Economy 57
- II.C.
-
Less Hazardous Chemical Syntheses 58
- II.D.
-
Reduce Use of Protecting Groups 58
- II.E.
-
Catalysis 62
- II.F.
-
Direct Isolations 62
- II.G.
-
Convergent Routes, Location of Low-Yielding Steps, and Resolutions64
- II.H.
Renuka Manchanayakage PhD, in, 2019