What Are The Advantages Of Crystallization
The key advantages of crystallization are listed below.
- A product of high purity can be obtained from one single step via the process of crystallization.
- The dry products formed from crystallization can be directly packaged and stored.
- The energy requirements and the operating temperatures of this process are relatively low.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Purification Methods For Organic Compounds
Purification methods for organic compounds very important for all chemist and engineers. We can do purification of organic compounds by method of distillation , extraction and much more. In this article we will study each method in details. Actually not details but we can do study up to basic level . I will come with each article on purification methods in some few days . Today we will cover introduction of main purification methods.
Some main purification methods are as below ,
- Liquid liquid extraction
- Column chromatography
Solved Examples For You
Question: Give two practical applications of simple crystallisation.
Answer: Practical applications of simple crystallisation include:
- Sugar having an impurity of common salt can be crystallized from hot ethanol since sugar dissolves in hot ethanol but common salt does not.
- A mixture of benzoic acid and naphthalene can be separated from hot water in which benzoic acid dissolves but naphthalene does not.
You May Like: What Is Figure Ground Perception Psychology
What Are The Uses Of Crystallization
Crystallization is primarily employed as a separation technique in order to obtain pure crystals of a substance from an impure mixture. Another important application of crystallization is its use to obtain pure salt from seawater. Crystallization can also be used to obtain pure alum crystals from an impure alum. In such scenarios, crystallization is known to be more effective than evaporation since it also removes the soluble impurities.
Buse Of Sfc As A Purification Tool In Discovery
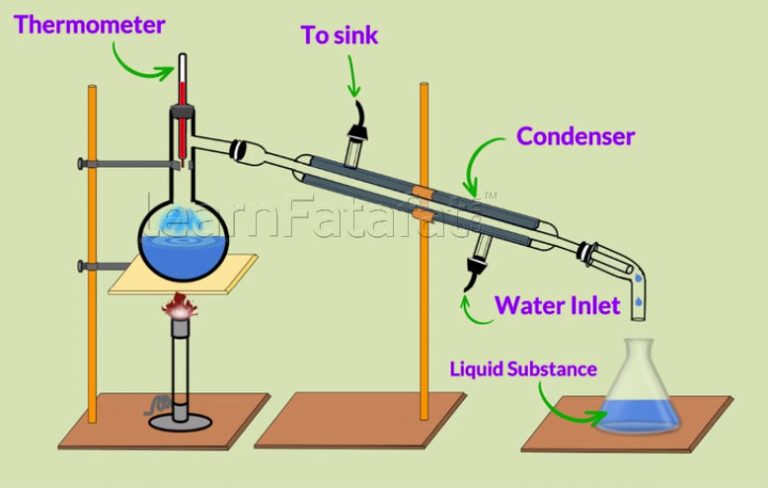
Discovery purification laboratories have embraced SFC technology8386 as a method of choice for chiral purifications over recent years but it has yet to gain traction as an impurity isolation tool with structure elucidation as the end goal. Few literature references report on this impurity application.69,8789 Discovery purification laboratories are in the business of achiral and chiral purifications on a milligram to multigram scale. SFC has gained explosive popularity, primarily focusing on chiral applications.9092 Implementation of SFC has resulted in increased productivity and throughput for these purification laboratories which, in turn, help expedite candidate decision-making processes. In addition to increased productivity, SFC delivers high-purity fractions with acceptable recoveries at significant solvent cost saving, resulting in substantial long-term operational cost savings. Preparative SFC has become so successful that Discovery purification laboratories have made a shift from preparative HPLC to preparative SFC as their primary method of choice for purification.
Yoshihiko Uratani, Toshimitsu Hoshino, in, 2000
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
Purification Methods Of Organic Compounds
Purification methods of organic compounds is carried out by number of ways . We can do purification of organic compounds by method of distillation , extraction and much more. In this article we will study each method in details. Actually not details but we can do study up to basic level . I will come with each article on purification methods in some few days . Today we will cover introduction of main purification methods.
Some main purification methods are as below ,
- Liquid liquid extraction
- Column chromatography
Distillation Under Reduced Pressure Or Vacuum Distillation
This method is used for the purification of high boiling liquids and liquid which decomposes at or below their boiling points.Principle: A liquid boils when its vapour pressure becomes equal to the external pressure. The same liquid would boil at a lower temperature if the pressure acting on it is reduced. Since the liquid now boils at a lower temperature, its decomposition does not occur. With vacuum pumps, pressure of the order of 0.1 mm Hg can be easily obtained.
For Example:
Glycerol which decomposes at its boiling point can be distilled without decomposition at 453 K under 12 mm Hg pressure.
Concentration of sugarcane in sugar industry.
Also Check: What Does Vertical Mean In Geometry
Purification Of Desired Polypeptide Segment
Purification of the final polypeptide segment away from the previously fused domain used in affinity purification is, in most cases, a repetition of the procedure for purification of the fusion protein. However, at this point the fraction which is saved is that which does not bind to the affinity column, rather than the one that does. Typically, these affinity procedures provide polypeptide of reasonable purity. Frequently, it is also necessary to subject the partially pure polypeptide to further chromatographic purification, particularly if the material is to be used for NMR analysis. One often-used method is to purify the polypeptide by ion exchange chromatography, or size exclusion chromatography . The purity of the polypeptide can be assessed at each stage by SDSPAGE analysis.
W. Shi, M.R. Chance, in, 2007
Reasons For Making Separations
There are two general reasons for performing separations on mixtures. First, the mixture may contain some substance that should be isolated from the rest of the mixture: this process of isolating and thus removing substances considered to be contaminants is called purification. For example, in the manufacture of synthetic drugs, mixtures containing variable proportions of several compounds usually arise. The removal of the desired drug from the rest of the mixture is important if the product is to have uniform potency and is to be free of other components that may be dangerous to the body.
Recommended Reading: Who Are Paris Jackson’s Biological Parents
Distinguishing Between Pure Substances And Mixtures
Pure substances have a sharp melting point but mixtures melt over a range of temperatures. This difference is most easily seen when the temperature of a hot liquid is measured as it cools and freezes. The graph shows the cooling curve for a sample of a compound called salol.
The horizontal part of the graph shows that the salol has a sharp melting point, so it is pure. Impure salol would produce a gradual decrease over a range of temperatures as it freezes.
List Of Purification Methods In Chemistry
Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. Pure results of a successful purification process are termed isolate. The following list of chemical purification methods should not be considered exhaustive.
You May Like: Structure Of Ccl4
Chemistry Behind Reverse Osmosis Water Purification
June 1, 2019 By scienceofwater
Water is the most essential component of earth and a medium that supports living. Unfortunately, increases in natural calamities, like floods and droughts and man-built water pollution has disturbed the water levels, leading in the scarcity of pure drinking water. Scientists and environmental experts are constantly searching for different water purification processes that can provide safe and pure drinking water for humanity. One of the most reliable tested, trialed and preferred water purification processes is Reverse Osmosis. It is worth and essential for the current user and future generations to have an in-depth insight into the chemistry behind reverse osmosis and its successful use in water purification.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Also Check: Does Elton John Have Biological Children
Ways To Purify Chemicals That Could Change The World
Most industrial chemists spend their days separating the components of large quantities of chemical mixtures into pure or purer forms. The processes involved, such as distillation, account for 1015% of the world’s energy consumption.
Methods to purify chemicals that are more energy efficient could, if applied to the US petroleum, chemical and paper manufacturing sectors alone, save 100 million tonnes of carbon dioxide emissions and US$4 billion in energy costs annually . Other methods would enable new sources of materials to be exploited, by extracting metals from seawater, for example.
Unfortunately, alternatives to distillation, such as separating molecules according to their chemical properties or size, are underdeveloped or expensive to scale up. Engineers in industry and academia need to develop better and cheaper membranes and other ways to separate mixtures of chemicals that do not rely on heat.
Here, we highlight seven chemical separation processes that, if improved, would reap great global benefits. Our list is not exhaustive almost all commercial chemicals arise from a separation process that could be improved.
Role Of Raoults Law And Daltons Law
The temperature at which the vapor pressure of a liquid becomes equal to the pressure of the surrounding area is known as the boiling point of that liquid. At this temperature point, the liquid is converted into its vapor form via the formation of vapor bubbles at its bulk.
It is important to note that the boiling point of the liquid changes with the surrounding pressure. For example, the boiling point of water at sea level is 100oC but its boiling point at an altitude of 1905 meters is 93.4oC .
For a mixture of liquids, the distillation process is dependent on Daltons law and Raoults law. As per Raoults law, the partial pressure of a single liquid component in an ideal liquid mixture equals the product of the vapor pressure of the pure component and its mole fraction. According to Daltons law of partial pressures, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of all the constituent gases.
When a mixture of liquids is heated, the vapor pressure of the individual components increases, which in turn increases the total vapor pressure. Therefore, the mixture cannot have multiple boiling points at a given composition and pressure.
Also Check: What Does Abiotic Mean In Biology
Basic Concepts Of Separations
This section is concerned with separations of the smallest subdivisions of matter, such as atoms, molecules, and minute particles . Such processes start with a sample in a mixed state and transform it into new samples, each of whichin the ideal caseconsists of a single substance. Separation methods, then, can be defined as processes that change the relative amounts of substances in a mixture. In chemical methods, one may start with a completely homogeneous mixture or a heterogeneous sample in the act of separation, some particles are either partially or totally removed from the sample.
Recrystallization And Purification Techniques For Getting A Pure Sample
Could someone explain in detail as to why exactly recrystallization gives a more pure solid sample? As well as why is would be better ‘technique’ to allow for the crystal to form slowly?
I was thinking that it would be because being a purification technique it essentially breaks the lattice and allows the structure to reform again. As for why it needs to be done slowly, well, because rapid changes in heat would cause the structure to reform into the impure solid it was.
I’m looking for a more detailed, better expressed, answer to this.
- $\begingroup$Why recrystallization gives a more pure solid sample than what? Precipitation? Sublimation? Evaporation? Fractional crystallization?$\endgroup$
Recrystallization is a purification method because it is a slow selective generation of a crystal framework, free of trapped impurities. It is only effective as a purification method if done properly. Poor recrystallization techniques rapid cooling, dramatic changes in solubility with temperature, bad choice of solvent, over evaporation of solvent, can all produce crystals of low purity which are not much better than the starting crop.
Impurities in crystals can occur via three mechanisms
Of course, there other methods for purifying solids which are effective also, if used correctly. Precipitation, sublimation and fractional crystallization are all methods for purifying solids, which can give high levels of purity, matching purities obtained be recrystallization.
You May Like: Geometry Dash Hack No Jailbreak
Different Types Of Chemical Substance
- an element contains just one type of atom
- a compound contains two or more types of atom joined together
- a mixture contains two or more different substances that are not joined together
- the different substances in a mixture can be elements or compounds
The table shows some examples:
Notice that the different substances in a mixture can be single atoms, molecules of elements or molecules of compounds.
What Is The Difference Between Filtration And Purification
Filtration is the technique of removing solids in a fluid via passing the fluid through a barrier which can hold the solid particles whereas purification is the technique of removing any unwanted particles from a sample in order to isolate the desired compound. Therefore, filtration is a form of purification technique that we can use to separate a solid from a fluid . The filtration gives a filtrate at the end of the process whereas purification gives an isolate. Therefore, this is the key difference between filtration and purification.
The below infographic presents the difference between filtration and purification in tabular form for quick reference.
Read Also: What Is The Molecular Geometry Of Ccl4
Purification Of Organic Compounds
Almost everything that we see these days is impure, isnt it? The water we drink and the food we eat also need to go through levels of purification processes. Similar is the case with organic compounds. There are several methods of purification of organic compounds. Why are these important and how do we do it? Let us learn all that in this chapter!
Why Is It Impossible To Completely Purify A Mixture By Distillation
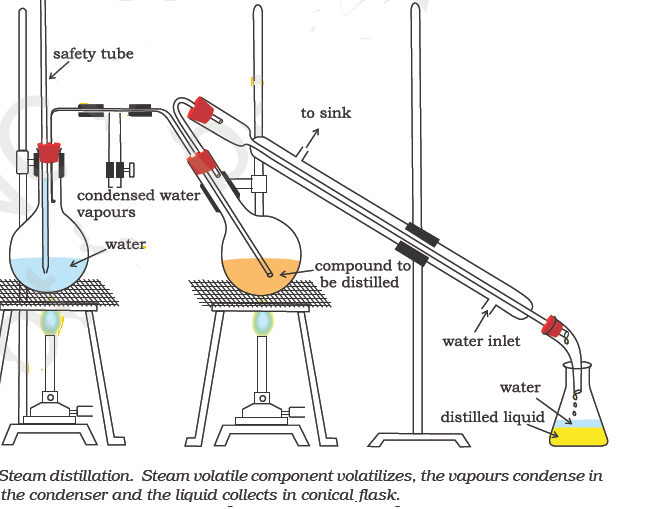
At the boiling point of a mixture of liquids, all the volatile constituents boil. However, the quantity of a constituent in the resulting vapor is based on its contribution to the total vapor pressure of the mixture. This is why the compounds with higher partial pressures can be concentrated in the vapors whereas the compounds having low partial pressures can be concentrated in the liquid.
Since a component in the mixture cannot have zero partial pressure, it is impossible to obtain a completely pure sample of a component from a mixture via distillation. However, samples of high purity can be obtained when one of the components in the mixture has a partial pressure which is close to zero.
You May Like: Lesson 4.5 Practice B Geometry Answers
Purification Of Colloidal Solutions
Stable & Unstable Colloidal Solution
The colloidal solution is the solution that has the particle size ranging from true solutions and suspensions. The range of the diameter of the dispersed particle is from 10 Angstrom to 2000 Angstrom.
Some of the methods of purification of a colloidal solution are stated below:
This was just a brief layout of some methods of purification of the colloidal solution. To know more in detail about the purification of suspension and colloids and surface chemistry, please visit BYJUS.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
What Is The Definition Of The Term Crystallization
Crystallization can be defined as the solidification of a liquid substance into a highly structured solid whose atoms or molecules are placed in a well-defined three-dimensional crystal lattice. The smallest individual part of a crystal is called a unit cell. The crystal is made up of millions of such unit cells.
Recommended Reading: My Hrw Com Algebra 1
Difference Between Filtration And Purification
September 14, 2018 Posted by Madhu
The key difference between filtration and purification is that filtration is a technique that separates solids from fluids via filtering off the fluid through a barrier whereas purification is a process of separating unwanted components from a fluid via different techniques such as filtration and disinfection.
Filtration is a purification technique in which we use a barrier through which a fluid can be filtered off. This removes the solid components in the fluid. Purification is a very broad term in which we can discuss various methods other than filtration that is useful in purifying a sample.
Summary Filtration Vs Purification
A filtration is a form of purification technique that we use to separate different components in a sample. The key difference between filtration and purification is that the filtration is a technique that separates solids from fluids via filtering off the fluid through a barrier whereas the purification is a process of separating unwanted components from a fluid via different techniques such as filtration and disinfection.
Reference:
1. Helmenstine, Anne Marie, Ph.D. Filtration Definition and Processes . ThoughtCo, Jun. 22, 2018. Available here 2. List of Purification Methods in Chemistry. Wikipedia, Wikimedia Foundation, 6 Aug. 2018. Available here
Image Courtesy:
1.FilterFunnelApparatusBy Smokefoot Own work, via Commons Wikimedia2.Affinity Chromatography TechniqueBy Tinojasontran via Commons Wikimedia
Read Also: Which Founding Contributors To Psychology Helped Develop Behaviorism
Protein Synthesis And Sequencing
Solid-Phase Protein Synthesis
Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains.Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide , and proceeds toward the amino-terminus . Protein biosynthesis in living organisms occurs in the opposite direction. Chemical synthesis facilitates the production of peptides which are difficult to express in bacteria, the incorporation of unnatural amino acids, peptide/protein backbone modification, and the synthesis of D-proteins, which consist of D-amino acids.
The established method for the production of synthetic peptides in the lab is known as solid-phase peptide synthesis . Pioneered by Robert Bruce Merrifield, SPPS allows the rapid assembly of a peptide chain through successive reactions of amino acid derivatives on an insoluble porous support.
Each amino acid to be coupled to the peptide chain N-terminus must be protected on its N-terminus and side chain using appropriate protecting groups such as Boc or Fmoc , depending on the side chain and the protection strategy used .
Figure 3.29 Solid-phase synthesis of a dipeptide using an amide resin. The N-terminal protecting group can be Fmoc or Boc, depending on the protecting group scheme used. The amino acid side chains are orthogonally protected.