What Are The Three Types Of Compounds
We all know that a chemical element has one type of atom only. When a substance contains more than one kind of atom, then we say that it is a compound. In other words, we can say that a compound refers to a substance in which two or more atoms are bonded with each other.
Millions of compounds exist and all fall in the following three broad categories:
1) Ionic Compounds These compounds are made up of ions. Ions are charged particles that are made when an atom gains or loses electrons. There are two types of ions: cation and anion. A cation is a positively charged ion and the anion is a negatively charged ion. These compounds are generally formed by a reaction between a metal and a nonmetal. To determine how to name these compounds, see the rules for naming ionic compounds in the previous section. 2) Molecular or Covalent Compounds They are formed when elements of the compound share electrons in a covalent bond to make up a molecule. These compounds are formed by the reaction between two nonmetals. 3) Acids Acids are compounds that contain hydrogen. It is easy to recognize acids as they contain hydrogen and anion. For instance, is named as nitric acid and is named as sulphuric acid.
Why Is Nomenclature Important What Is The Purpose Of Nomenclature
There are two objectives of using nomenclature in chemistry:
- To make sure that a spoken or written chemical name does not contain any ambiguity regarding the chemical compound the name is referring towards. It is important that each chemical name points towards a single substance.
- To ascertain that each substance has one name only
- To help the chemists communicate with their peers easily.
Emma
What Is The Difference Between An Ide And A Text Editor
IDE stands for Integrated development environment not just a tool where you write the code, but you can also compile it and debug it.. text editors in their nature, usually dont do that, they tend to go for a broader approach.. be able to edit all types of files, instead of specializing in a particular type or
Read Also: Who Is Paris Jackson Parents
Naming Ions And Ionic Compounds
Ionic compounds consist of cations and anions .The nomenclature, or naming, of ionic compounds is based on the names of the component ions. Here are the principal naming conventions for ionic compounds, along with examples to show how they are used:
Roman NumeralsA Roman numeral in parentheses, preceded by the name of the element, is used for elements that can form more than one positive ion.
This is usually seen with transition metals.Fe2+ Iron Cu2+ Copper
-ous and -icAlthough Roman numerals are used to denote the ionic charge of cations, it is still common to see and use the endings -ous or -ic. These endings are added to the Latin name of the element to represent the ions with lesser or greater charge, respectively.The Roman numeral naming convention has wider appeal because many ions have more than two valences.Fe2+ Ferrous
The -ide ending is added to the name of a monoatomic anion of an element.H- Hydride
P3 Phosphide
-ite and -ateSome polyatomic anions contain oxygen. These anions are called oxyanions. When an element forms two oxyanions, the one with less oxygen is given a name ending in -ite and the one with more oxygen is given a name that ends in -ate.NO2- NitriteSO4- Sulphate
hypo- and per-In the case where there is a series of four oxyanions, the hypo- and per- prefixes are used in conjunction with the -ite and -ate suffixes. The hypo- and per- prefixes indicate less oxygen and more oxygen, respectively.ClO- HypochloriteClO4- Perchlorate
Chambers 20th Century Dictionaryrate This Definition:
Oxide
oksd, n. a compound of oxygen and some other element or organic radical. Oxides are of three kindsacid-forming, basic, and neutral.n.Oxidability.adj.Oxidable, capable of being converted into an oxide.v.t.Oxidate .ns.Oxidtion, Oxidisement, act or process of oxidising Oxidtor, a contrivance for drawing a current of air to the flame of a lamp.adj.Oxidisable, capable of being oxidised.v.t.Oxidise, to convert into an oxide.v.i. to become an oxide.n.Oxidiser.
You May Like: Holt Geometry Lesson 4.5 Practice B Answers
How Long Does Fda Ide Approval Take
IDEs must be considered approved thirty days after being received by the FDA, without the FDA communicating with the sponsor via email prior to that date, that the IDE will be submitted to FDA if conditions are met, that it will meet or qualify for approval or have already been developed in this way
Compounds Between Metals And Nonmetals
Compounds made of a metal and nonmetal are commonly known as Ionic Compounds, where the compound name has an ending of ide. Cations have positive charges while anions have negative charges. The net charge of any ionic compound must be zero which also means it must be electrically neutral. For example, one Na+ is paired with one Cl- one Ca2+ is paired with two Br-. There are two rules that must be followed through:
- The cation is always named first with its name unchanged
- The anion is written after the cation, modified to end in ide
| +1 Charge |
|---|
You May Like: Elton John Kids Adopted
What Does Ous Mean In Chemistry
4.8/5ousous
Then, what is the difference between IC and ous?
The transition metals often form two or more different monoatomic cations. An older nomenclature for distinguishing between the different ions of a metal is to use the suffixes –ous and –ic. The suffix –ic will indicate the ion of higher ionic charge: Fe3+ ferric ion.
One may also ask, what does the IDE ending mean in chemistry? Most end with the syllable “ide” and they use the Greek prefix to indicate the number of atoms. The name of second element is shortened and the suffix “ide” is added. If more than one atom of the second element, then use the Greek prefix for that number. Here I’m talking chemistry to a couple of textbooks authors.
Keeping this in consideration, what does IC mean in chemistry?
–ic. Used to form adjectives from nouns with the meaning of or pertaining to. Cyrillic acidic. Used to denote certain chemical compounds in which a specified chemical element has a higher oxidation number than in the equivalent compound whose name ends in the suffix -ous.
How do you know when to use IDE or ate in chemistry?
The –ide ending is added to the name of a monoatomic anion of an element. Some polyatomic anions contain oxygen. These anions are called oxyanions. When an element forms two oxyanions, the one with less oxygen is given a name ending in -ite and the one with more oxygen is given a name that ends in –ate.
Naming Acids
What Are The Types Of Ide
Different Types of IDE
- Eclipse: Supports C, C++, Python, Perl, PHP, Java, Ruby and more.
- NetBeans: Supports Java, JavaScript, PHP, Python, Ruby, C, C++ and more.
- Komodo IDE: Supports Perl, Python, Tcl, PHP, Ruby, Javascript and more.
- Aptana: Supports HTML, CSS, JavaScript, AJAX and others via plugins.
Recommended Reading: How To Calculate Half Life Chemistry
Rules For Naming Ionic Or Molecular Compounds
Here are the simple steps to name compounds in chemistry: Step 1: Determine whether the compound in an ionic or molecular compound The first step is to identify whether the compound you are going to name is an ionic compound or a molecular compound. To do so, you should know what ionic and molecular compounds are.
- The compound is ionic if it contains a metal. Metals are present on the middle and left side of the periodic table.
- The compound is molecular if it contains two nonmetals. Nonmetals are present on the right side of the periodic table above the staircase, including hydrogen)
| mono |
|---|
Why Do Developers Use Ides
An IDE allows developers to start programming new applications quickly because multiple utilities dont need to be manually configured and integrated as part of the setup process. Developers also dont need to spend hours individually learning how to use different tools when every utility is represented in the same workbench. This can be especially useful for onboarding new developers who can rely on an IDE to get up to speed on a teams standard tools and workflows. In fact, most features of IDEs are meant to save time, like intelligent code completion and automated code generation, which removes the need to type out full character sequences.
Other common IDE features are meant to help developers organize their workflow and solve problems. IDEs parse code as it is written, so bugs caused by human error are identified in real-time. Because utilities are represented by a single GUI, developers can execute actions without switching between applications. Syntax highlighting is also common in most IDEs, which uses visual cues to distinguish grammar in the text editor. Some IDEs additionally include class and object browsers, as well as class hierarchy diagrams for certain languages.
Today, most enterprise development teams opt for a pre-configured IDE that is best suited to their specific use case, so the question is not whether to adopt an IDE, but rather which IDE to select.
You May Like: Holt Mcdougal Geometry Worksheet Answers
Popular Kinds Of Ides
There are many different technical and business use cases for IDEs, which likewise means there are many proprietary and open source IDE options on the market. Typically, the most important differentiating characteristics between IDEs are:
- The number of supported languages: Some IDEs are dedicated to one language, and so are a better match for a specific programming paradigm. IntelliJ, for instance, is known primarily as a Java IDE. Other IDEs have a broad array of supported languages all in one, like the Eclipse IDE which supports Java, XML, Python, and others.
- Supported operating system: A developers operating system will constrain which IDEs are viable , and if the application being developed is intended for an end user with a specific operating system , this may be an additional constraint.
- Automation features: Even though most IDEs include the 3 key features of a text editor, build automation, and debugger, many include support for additional features like refactoring, code search, and continuous integration and continuous deployment tools.
- Impact on system performance: An IDEs memory footprint may be important to consider if a developer wants to run other memory-intensive applications concurrently.
- Plugins and extensions: Some IDEs include the ability to customize workflows to match a developers needs and preferences.
Which One Is Stronger Ionic Or Covalent Bond

Ionic Bonds are stronger than covalent bonds because the electronegativity difference between the two elements is much greater than that of two elements in a covalent bond. Covalent bonds allow the electrons to be shared between the two elements and will often favor one element over the other depending on polarity.
Don’t Miss: Kuta Software Infinite Algebra 2 Solving Inequalities Answers
Naming Ionic Compounds Using Hypo
In the case where there is a series of four oxyanions, the hypo- and per- prefixes are used in conjunction with the -ite and -ate suffixes. The hypo- and per- prefixes indicate less oxygen and more oxygen, respectively.
- ClO- Hypochlorite
- ClO3- Chlorate
- ClO4- Perchlorate
Example: The bleaching agent sodium hypochlorite is NaClO. It is also sometimes called the sodium salt of hypochlorous acid.
How Do You Know When To Use Ide Or Ate
When naming molecular compounds prefixes are used to dictate the number of a given element present in the compound. For example: and deca is ten. For a more in depth explanation check out this video. How do you know whether to use ‘ide’ or ‘ate’, when naming a compound? -ide is used for non-metal compounds generally.
Also Check: Altogether Math Definition
What Is Ide In Oxide
A binary compound of a nonmetallic or electronegative element. German -id, taken from French oxide, an oxide, created by analogy with the ending of acide, acid. The ending is also used to denote compounds that are derivatives of organic radicals: peptide, saccharide, amide, anhydride, cyanide, anilide, etc.
Ionic Compounds Containing Bi
Polyatomic anions sometimes gain one or more H+ ions to form anions of a lower charge. These ions are named by adding the word hydrogen or dihydrogen in front of the name of the anion. It is still common to see and use the older naming convention in which the prefix bi- is used to indicate the addition of a single hydrogen ion.
- HCO3- Hydrogen carbonate or bicarbonate
- HSO4- Hydrogen sulfate or bisulfate
- H2PO4- Dihydrogen phosphate
Example: The classic example is the chemical name for water, H2O, which is dihydrogen monoxide or dihydrogen oxide. Dihydrogen dioxide, H2O2, is more commonly called hydrogen dioxide or hydrogen peroxide.
You May Like: Redken Acidic Bonder Vs Olaplex
What Does The Suffix Ite Mean In Chemistry
4.8/5suffixite suffixmeans
People also ask, what do the endings ITE and ate indicate?
The name of the ion usually ends in either –ite or –ate. The –ite ending indicates a low oxidation state. Thus,the NO2- ion is the nitrite ion. The –ate ending indicates a high oxidation state.
Similarly, what does the suffix IDE mean in chemistry? –ide. A suffix used to form the names of various chemical compounds, especially the second part of the name of a compound that has two members or the name of a general type of compound .
Regarding this, what does suffix mean in chemistry?
The suffix to the name is an ending that is applied that describes the types of chemical bonds in the molecule. An IUPAC name also includes the names of substituent groups that make up the molecular structure.
What does ate at the end of a compound mean?
If the compound contains three elements one of which is oxygen then the compound name will end in ate or ite, eg Calcium carbonate contains calcium, carbon and oxygen.
What Does Ate Ite And Ide Mean In Chemistry
-ide is used for non-metal compounds generally. For example, Chlorine forms a chloride ion, so NaCl is Sodium Chloride. -ate and -ite are commonly used for polyatomic ions of Oxygen. -ate is used for the ion that has the largest number of Oxygen atoms. The -ite would be used for the ion with the smaller.
Recommended Reading: What Happened To Beth Thomas Brother Jonathan
What Are The General Rules For Nomenclature What Are Nomenclature Rules
Scientists employ nomenclature to name compounds clearly in chemistry. Ionic and molecular compounds are named using distinct methods. Nomenclature in chemistry refers to a set of rules to generate systematic names of compounds. The nomenclature which is used by the chemists and scientists worldwide is created and developed by the IUPAC .
The Old Classic Or Common Way Of Naming

Names of some ionic compounds: Common, or trivial, names of compounds are sometimes used in informal conversations between chemists, especially older chemists. Systematic names are formal names that are always used in print.
Since some metallic elements form cations that have different positive charges, the names of ionic compounds derived from these elements must contain some indication of the cation charge. The older method uses the suffixes -ous and -ic to denote the lower and higher charges, respectively. In the cases of iron and copper, the Latin names of the elements are used . This system is still used, although it has been officially supplanted by the more precise, if slightly cumbersome, Stock system. In both systems, the name of the anion ends in -ide.
Naming Compounds Part 1 YouTube: This video explains how to name covalent and ionic compounds.
Read Also: What Type Of Math Do Architects Use
Writing Formulas Of Ionic Compounds
Remember thePrime Directive in writing formulas:Ca2 ¹ CaOH2 !
What Are Anions Used For
Anions are negatively charged ions, and are formed from atoms or molecules that have more electrons than protons. Anions often combine with cations to make salts, which are important in the human body. These particles play a role in many vital biological processes, from hormone production to DNA formation.
Recommended Reading: Beth Thomas Father
How Do You Identify Types Of Compounds
You can identify the type of compound by simply looking at the nature of its composition. Ionic Compounds: These compounds are formed when metal and non-metal are joined together. If you see that a compound is made from a metal and nonmetal, then you can easily categorize it as an ionic compound. For instance, NaCl is an ionic compound because sodium is a metal and chlorine is a nonmetal. Covalent compounds: These compounds are formed when two nonmetals are held together by a covalent bond. When you see a compound with two or more nonmetals, then you can easily term it as a covalent compound. For instance, carbon monoxide is made from two nonmetals carbon and oxygen, hence it is a covalent compound Acids: Acids contain hydrogen and anion.
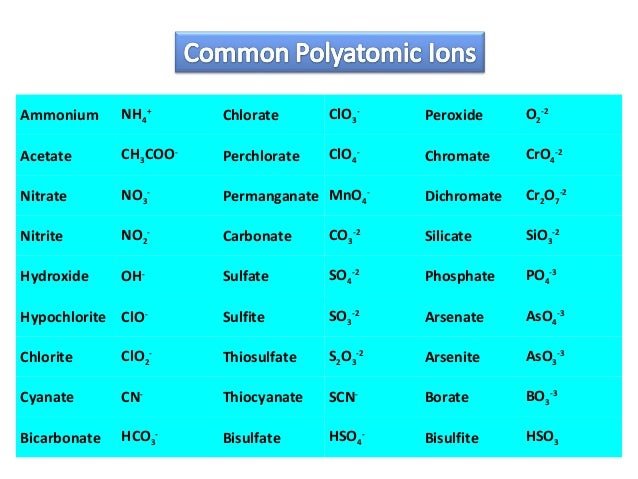
Naming Ionic Compounds That Contain Polyatomic Ions
The rules for naming ionic compounds containing polyatomic ions are different. Polyatomic ions contain more than one atom. For instance, has one nitrogen atom and four oxygen atoms. In a polyatomic ion, the atoms are generally covalently bonded to each other. They act as a single charged unit. Most of the compounds containing polyatomic ions end with “ate” or “ite”. Only some of them end with “ide”. For instance, is named as sodium sulphate and is called sodium sulphite.
Also Check: Geometry Basics Segment Addition Postulate Worksheet Answers