Determining The Flash Point And Auto
To determine the flash point and auto-ignition temperature of your flammable liquid, you should always refer to the Safety Data Sheet of your individual chemical product. You will find this information in the physical and chemical properties section of your SDS, along with other important information including the chemicals physical state, colour, odour, melting point, boiling point and flammability.
Refer to the SDS of your individual chemical products to determine the flash point and auto-ignition temperature of your flammable liquids.
Remember that every type of flammable liquid will have a particular flash point and auto-ignition temperature. Staff should always have access to the SDS and be trained in hazard prevention through flammable liquids safety training.
Facts About Flammable And Combustible Liquids
- Flammable and combustible liquids ignite easily and burn with extreme rapidity.
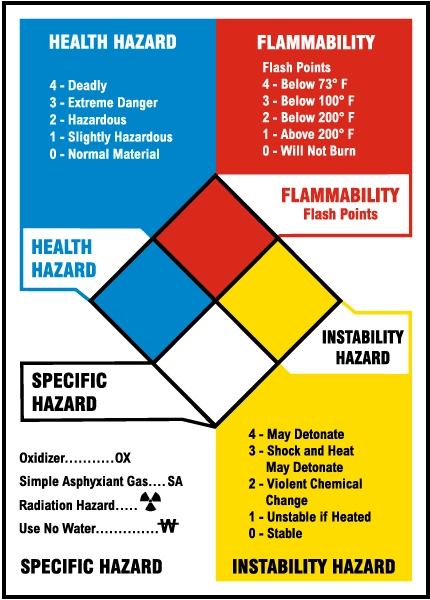
- Flammability is determined by the flash point of a material.
- Flash point is the minimum temperature at which a liquid forms a vapor above its surface in sufficient concentration that it can be ignited.
- Flammable liquids have a flash point of less than 100°F. Liquids with lower flash points ignite easier.
- Combustible liquids have a flashpoint at or above 100°F.
- The vapor burns, not the liquid itself. The rate at which a liquid produces flammable vapors depends upon its vapor pressure.
- The vaporization rate increases as the temperature increases. Therefore, flammable and combustible liquids are more hazardous at elevated temperatures than at room temperature.
- Class 1 Flammable Liquids must be bonded and grounded when transferring liquids.
Petrol Fire Hyderabad India
The driver of a black sedan was lucky to avoid disaster as his Skoda Superb caught on fire at the fuel pump.
Having left his vehicle while it was refuelling, the car burst into flames which spread through the handpiece and hose to the gas station.
NDTV reported that petrol spilled over during the refuelling, with the heat from the vehicle causing the fire. Emergency crews reached the scene quickly and were able to contain the fire.
Read Also: What Does Amu Stand For
Transportation And Storage Regulations Hazard Classification
Flash point is used in shipping and safety regulations to define flammable and combustible materials and classify their hazard potential which has significant cost implications when transportingor storing products.
Many industries use solvents in their products which are used to classify the flash point for the finished product. Some solvents are not highly flammable so establishing the exact flash point can help save money.
The Setaflash test method in these circumstances quickly provides an accurate flash point value to correctly classify the true hazard nature of a product and uses a small sample size.
Examples Of Flash Point In A Sentence

flash pointflash pointflash point Forbesflash point Anchorage Daily Newsflash point Los Angeles Timesflash point ABC Newsflash point BostonGlobe.comflash point New York Timesflash point Washington Postflash point Anchorage Daily News
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘flash point.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Also Check: Geometry Worksheet Answers Mcdougal Littell
Flash Point Testing Methods
There are two main methods for carrying out the flash point test: open cup and closed cup.
Each of these methods may be more appropriate for different applications. Certain products or regulations may specify one method or the other.
The type of product you are testing should be within the scope of the testing method you choose.
Within the two broad open cup and closed cup classes of flash point testing, there are several different techniques.
Fire Point Vs Flash Point
Contrast this with the flash point, which is a lower temperature at which a substance will ignite, but may not continue to burn.
The fire point for a specific fuel is not typically listed, while flash point tables are readily available. Generally, the fire point is about 10 °C higher than the flash point, but if the value must be known, it should be determined experimentally.
You May Like: Did Michael Jackson Have Biological Children
Difference Between Flash Point And Fire Point
April 30, 2011 Posted by Madhu
The key difference between flash point and fire point is that the flash point describes the lowest temperature at which the ignition of a substance initiates whereas the fire point describes the lowest temperature at which the fuel continues to burn for a short time period after the initiation of the ignition.
All flammable liquids have a vapour pressure that increases with its temperature. The concentration of evaporated liquid in the air increases with an increase in vapour pressure. Different flammable liquids require different concentrations in the air to sustain combustion. Here, the flash point of a flammable liquid is the lowest temperature at which it can form an ignitable mixture in air. However, the vapour stops to burn if we remove the source of ignition. Whereas, the fire point is the temperature at which vapours of the flammable liquid continue to burn after being ignited even after we remove the source of ignition. However, both the flash point and fire point have no relation to the temperature of the source of ignition.
The Flash Point Of A Chemical Indicates How Easy It May Ignite And Burn
The flash point of a chemical is the lowest temperature where it will evaporate enough fluid to form a combustible concentration of gas. The flash point is an indication of how easy a chemical may burn.
Materials with higher flash points are less flammable or hazardous than chemicals with lower flash points.
| Hazard |
|---|
| < 0oF |
An open flame is not always necessary to ignite a gas. A hot surface – like a heating element or warm machine – will do for chemicals with more than high hazard.
The Flash Point is not the same as the Auto-Ignition Temperature. The Auto-Ignition Temperature is the minimum temperature required to ignite a gas or vapor in air without a spark or flame present.
- Risk, Reliability and Safety – Risk, reliability and safety in process control systems.
- Material Properties – Material properties of gases, fluids and solids – densities, specific heats, viscosities and more.
Read Also: Why Was The Pail Pale Answers
What Is Flash Point Testing
Flash point testing is a procedure designed to determine whether a sampled mixture of vapour and air is flammable. It can also determine the temperature at which flammability occurs in a sample.
The lowest temperature at which its vapours ignite from an ignition source is the flash point of a material.
Flash point analysis is important for testing various products, including lubricants and petroleum-based materials. The oil industry uses flash point testing for oil analysis.
Open Cup Flash Point Measurement
The open cup method for flash point testing uses a vessel, or container, that is exposed to the outside air.
Once the sample material is placed in the vessel, you then gradually raise its temperature, and pass an ignition source over it, until it flashes and ignites at a certain point.
This is the samples flash point.
The most common open cup method is the Cleveland open cup . Other methods include Tag and Setaflash.
Initially, the open cup method for flash testing was developed to assess potential hazards when there were spillages of liquids.
As a method, the open cup test is less precise than closed cup, because vapours are free to escape into the atmosphere, and may be affected by local conditions.
You May Like: Automatic Processes Definition Psychology
What Is The Difference Between Flash Point And Fire Point
The flash point and fire point are two very important features of fuels. These terms describe the initiation and the continuity of the combustion of a fuel. Hence, the key difference between flash point and fire point is that the flash point describes the lowest temperature at which the ignition of a substance initiates whereas the fire point describes the lowest temperature at which the fuel continues to burn for a short time period after the initiation of the ignition. Furthermore, we can find a difference between flash point and fire point based on their values as well. That is the fire point is always a higher value than the flash point. In general fire, point is about 10 degrees higher than flash point of flammable liquids.
Biodiesel Fire Missouri Usa
A fire-storey column holding biodiesel at a Missouri stockyard holding biodiesel caught fire, with the top level engulfed in flames.
The fire department suggested that the blaze may have started when the biodiesel residue ignited after being heated by the sun.
News-Press Now stated that members of the fire crew suffered minor burns and one firefighter was treated for heat exhaustion.
Read Also: How To Beat Theory Of Everything 2
Flash Points And The Chemistry Behind Hazardous Materials
When thinking about the best way to transport hazardous materials, the first question we have is what makes this hazardous? Knowing what is hazardous about a given material informs our ability to protect against it becoming a hazardous situation for those that come into contact with it. Generally when dealing with industrial chemicals, the biggest hazard one has to deal with is flammability, and flammability of chemical substances is determined largely by their respective flash points. In order for you to understand what drums and containers you would like to purchase from Incineration Recycling Services, wed like to educate you a little bit more on flash points and why theyre important to consider in incineration recycling.
What is a flash point?
A flash point is the lowest temperature at which a volatile chemical substance will begin to ignite. However, this does not mean the point at which a substance will ignite on its own, but with an ignition source. For example, ethanol has a flash point that is lower than room temperature, which is why you can light certain alcoholic beverages on fire. However, its autoignition temperature is much higher than that, which we know because bottles of alcohol dont spontaneously burst into flames.
Why do flash points matter?
Storing Flammable Liquids Safely
Hazardous goods storage is a serious workplace issue which needs to be taken seriously by your staff. Its important to understand flammable liquids and their flash points, so you can make sure that theyre handled and stored in the appropriate manner. As a rule of thumb, flammable liquids should be stored in cool, dry, ventilated and secure areas to minimise the potential for fire or explosion. If youd like to find out if your dangerous goods storage systems are safe and compliant, download our free eBook Outdoor Dangerous Goods Storage Checklist by clicking the image below. Download our eBook and read it today.
Read Also: Geometry Dash Hacks No Survey
Manual Or Automated Flash Point Testing
In manual flash testing, the operator is in control throughout, monitoring, stirring and setting temperatures. The operator determines whether a flash has occurred during the test.
The alternative is automated flash testing, where electronics, software and mechanical actions mimic the operators actions. This can reduce operator time and save on resources.
What Is A Flash Point
As we have discussed, the flash point of a flammable liquid is the lowest temperature at which vapours of that liquid will ignite when close to an ignition source.
As temperatures increase, and flammable fuels become more gaseous, they will generally give off certain levels of vapour. When there is sufficient vapour to ignite, the flash point has been reached.
Therefore, with the potential for ignition, safety is paramount when youre considering the storage of flammable liquids. Everyone in your team, and particularly those responsible for WH& S, need to be educated about the flash points of fuels.
Unless all staff are paying close attention to the flash points of these substances, there is the very real threat of fires and explosions.
To get you started, weve collated some common examples of flammable liquids and their flash points from lowest to highest. Read on to learn more about the hazards associated with flammable goods and their respective flash points.
Read Also: What Influence Did Geography Have On Greek Society
Summary Flash Point Vs Fire Point
Flash point and fire point are important characteristic parameters for flammable or combustible liquids. The key difference between flash point and fire point is that the flash point describes the lowest temperature at which the ignition of a substance initiates whereas the fire point describes the lowest temperature at which the fuel continues to burn for a short time period after the initiation of the ignition.
Reference:
1. Helmenstine, Anne Marie, Ph.D. Fire Point Definition. ThoughtCo, Jun. 22, 2018. Available here2. Flash Point. Wikipedia, Wikimedia Foundation, 18 Sept. 2018. Available here
Image Courtesy:
1.Flaming cocktailsBy Nik Frey via Commons Wikimedia 2.Fire Point Sign by George Hodan via PublicDomainPictures.net
What Is The Difference Between Flash Point And Boiling Point
Flash point is the lowest temperature at which vapour of the material will ignite when given an ignition source. Boiling point is the temperature at which the vapour pressure of a liquid equals the external pressure surrounding the liquid. So, the key difference between flash point and boiling point is that every liquid has a boiling point, but only volatile liquids have a flash point.
Moreover, at the flash point of a liquid, we can observe ignition above the liquid while at the boiling point, we can observe the formation of bubbles inside the liquid. Hence, this is a notable difference between flash point and boiling point. If we look at their mechanisms, ignition of flammable vapour occurs in the presence of an ignition source at flash point, when there is enough vapour to induce ignition. However, at boiling point, the vapour pressure of a liquid becomes equal to the external pressure surrounding the liquid.
Don’t Miss: Geometry Basics Segment Addition Postulate Worksheet Answers
Testing Methods For Flash Point And Auto
Class 3 liquids are tested for their flammability using the open and closed-cup test, through the introduction of an ignition source at a particular temperature. However, the auto-ignition range of a chemical is tested by placing it in a small vessel which is then transferred to a temperature-controlled oven. Through controlling the temperature of the oven, you can determine the heat at which the chemical spontaneously ignites. This provides the auto-ignition temperature of the substance.
What Are The Uses Of Flash Point Testing

The major uses of flash point testing are:
- Assessing safety hazards of liquids and semi-solids, including their flammability
- Classifying these materials according to these results.
In assessing the risk of flammability, flash point analysis is an effective and efficient method.
Essentially, the lower a substances flash point temperature, the higher its risk of flammability.
Specifications of different materials will include their flash points to control this flammability risk, and for quality control purposes.
Correct classification of materials, including chemicals containing petroleum, is essential for maintaining up to date safety instructions about packaging, handling and use.
Flash point testing can determine whether a liquid is classifiable as flammable, ignitable or combustible. For example, a liquid with a flash point below ambient temperature will be more hazardous.
If there is a change in flash point in a material, this can indicate that it contains contaminants which could be volatile. Sometimes one material is adulterated by another, and flash point testing will expose this too.
Contaminants can have a significant effect on flash points, especially in those situations where the contaminant is more volatile than the material itself.
Another important use of flash point testing for contamination is in oil analysis.
Also Check: Who’s Khloe Kardashian’s Real Dad
What Are The Differences Between The Two
Because closed cup methods are conducted in a confined environment, the results are less likely to be interfered with by outside elements found in the laboratory. Furthermore, they understandably generally produce lower flash points, since the heat is contained and the substance is more likely to become flammable at an earlier stage. Because of this, they are generally used as industry standards, because they deliver lower results, which ensure safer practices.
However, it is important to note that neither open cup nor closed cup flash points are fundamental parameters belonging to the substance, but rather empirical results of testing. They will be affected by all manner of criteria, including the laboratory they are conducted in, the equipment used and the method of testing selected.
Related stories
What Is The Difference Between Flash Point And Ignition Point
There are different ways of testing volatile, flammable materials. Flash point is one, and another is ignition or autoignition point.
There is a key difference between what the two methods measure:
- Ignition point is the lowest temperature that a material vapourises into a gas and then ignites without any external ignition source or flame
- Flash point is the lowest temperature the vapour will ignite when exposed to an ignition source or flame.
The test for ignition point is different to flash point testing. To test for ignition point, you heat a vessel containing the sample material in an enclosed oven. You the measure the point where there is ignition of the sample.
This test is known as ASTM E659, the standard test method for autoignition.
Which test you choose to apply will depend on the type of material you are testing and its end application.
Read Also: How To Solve For Half Life
Flammable Liquids: Difference Between Flash Point And Auto
Originally published February 28, 2022 10:49:37 PM, updated February 28, 2022
When you work with Class 3 Flammable Liquids, its important to understand the chemical and physical properties of the specific product that youre using. Flammable liquids can ignite and explode, causing damage to people, property and the environment. Two important considerations when studying the Safety Data Sheet of your chemicals is the flash point and the auto-ignition temperature of your flammable liquids. In this blog, we look more closely at the difference between the flash point and auto-ignition temperature for Class 3 liquids. Well also explain some risk control measures that you can implement to ensure that your liquid chemicals are handled and stored in a safe way.
But first, well give the definition of a flammable liquid as provided by the Australian Dangerous Goods code.