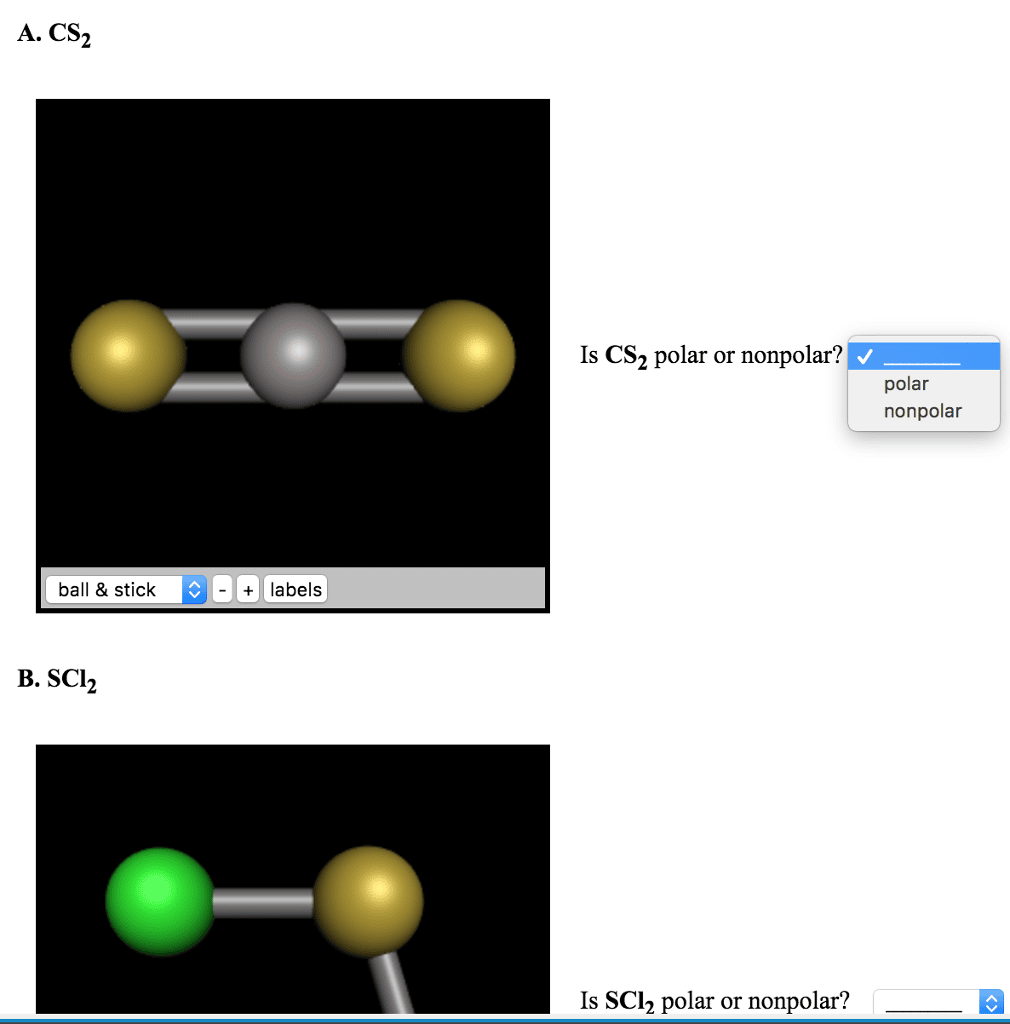
What Vsepr Shape Is Scl2
Sulfur is the central atom in the Lewis structure of SCl2 that has a steric number equal to 4, hence the electron geometry of SCl2 is tetrahedral. The molecular geometry of SCl2 is simply determined by considering only the number of bonded atoms, and as per VSEPR theory, its shape is bent.
Is VSEPR a model?
The VSEPR model is a model which predicts the geometrical shapes of molecules based on the repulsion between their lone pairs. Types of VSEPR structures include linear, trigonal planar and tetrahedral.
Is SCl2 a linear shape?
SCl2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm .
Sulfur Dichloride Polarity: Is Scl2 Polar Or Non
If you have basic knowledge of polarity, dipole moment, and electronegativity then you can already know the answer of Is SCl2 polar or non-polar?
SCl2 is polar because of its asymmetrical shape and non-uniform charge distribution around the atoms.
Also check-
To understand the depth of polar nature of SCl2 we have to take an overview of electronegativity, dipole moment, and geometrical shape.
1.Electronegativity
This is the big factor in knowing the polarity of SCl2 as the difference between the electronegativity of atoms is directly proportional to the polarity of molecules.
Electronegativity of chlorine = 3.16
Electronegativity of sulfur = 2.58
And the difference of these atoms makes electronegativity greater than 0.5 which directly makes SCl2 polar in nature.
2. Dipole moment
This factor can help us to know the strength of polarity. As greater the dipole moment more is the polarity of the molecule.
As SCl2 shape is asymmetric in nature. Because of this dipole moment of SCl2 does not cancel each other.
Dipole moment formula = charge on the atoms * the distance between them
D = Q × R
Net dipole moment of SCl2 is = 0.54d
3. Geometrical structure
The shape is also a big factor to determine whether SCl2 is polar or non-polar
More the asymmetrical shape of molecules greater is the dipole moment as symmetrical structure dipole moment can easily be canceled out but asymmetrical or bent structure provides some dipole moment which makes to molecules polar in nature.
What Is Vsepr Formula
The AXE method of electron counting is commonly used when applying the VSEPR theory. The electron pairs around a central atom are represented by a formula AXnEm, where A represents the central atom and always has an implied subscript one. Each X represents a ligand .
What is the shape of SCl4?
SCl4 has a seesaw molecular geometry because you must take into account the effect that the lone pair on S has on shape if there was no lone pair on SCl4, the shape would be tetrahedral.
What shape is PF3?
The VSEPR shape of the molecule PF3 is trigonal pyrimidal.
What is the VSEPR model used for?
Valence shell electron pair repulsion theory, or VSEPR theory , is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie- Nyholm theory after its two main developers,
What is the molecular geometry for SCl2?
The molecular geometry and polarity of sulfur dichloride, scl2 using vsepr rules is bent electron octahedral has a tetrahedral electronic geometry, eg because there are 4 different hybridized orbitals, we draw lewis structures to predict the shape molecule. The geometry name is bent .
What is the hybridization of SCl2?
Hybridization Of SCl2 In SCl2, the sulfur atom contains two bonding and lone pairs, which doesnt require pi bonding. Therefore the hybridization of SCl2 is sp3. Here is the definition of bond pairs and lone pairs.
Read Also: How To Do Good In Physics
Scl2 Lewis Structure Molecular Geometry Hybridization Polar Or Nonpolar
SCl2 Molecule
Sulfur dichloride is light amber to yellowish red, fuming, oily liquid with a strong, nauseating irritating odor. It is mostly used in organic synthesis and its chemical formula is SCl2.
It can be prepared by reacting sulfur with dichloride, first form disulfur dichloride. Each disulfur dichloride further reacts with dichloride then sulfur dichloride is formed.
Properties of SCl2 molecule
- The molecular mass of SCl2 is 102.97 g/mol.
- The boiling of SCl2 is 59°C and the melting point is -121°C.
- SCl2 has a density of 1.621 g/cm3 and a refractive index of 1.5570.
| Compound Name | |
| Is SCl2 Polar or Nonpolar? | Polar |
Uses of SCl2 molecule
- It is used for hardening softwoods.
- It is used as an intermediate and chlorinating agent in the manufacture of organic chemicals, sulfur dyes, insecticides, and synthetic rubber.
- It is used in insecticides and makes vulcanized oils.
- It is used to purify sugar juices.
- It is used as a chlorinating agent in various industries.
How Do You Find The Bond Angle
The Cl-S-Cl bond angle is 103 degrees in the tetrahedral SCl2 molecular geometry. The SCl2 molecule has a tetrahedral geometry shape because it contains two chlorine atoms in the tetrahedral and two corners with two lone pairs of electrons. There are two S-Cl single bonds at the SCl2 molecular geometry.
Recommended Reading: Accredited Online Algebra 1 Course
Drawing The Lewis Structure For Scl2
Video: Drawing the Lewis Structure for SCl2
For the SCl2 Lewis structure use the periodic table to find the total number of valence electrons for the SCl2 molecule. Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center.
For the SCl2 Lewis structure we have a total of 20 valence electrons.
It is helpful if you:
- Try to draw the SCl2 Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to SCl2 for more practice.
What Customers Are Saying:
Wonderful service, prompt, efficient, and accurate. Couldn’t have asked for more. I cannot thank you enough for your help.
Mary C.Freshfield, Liverpool, UK
This expert is wonderful. They truly know what they are talking about, and they actually care about you. They really helped put my nerves at ease. Thank you so much!!!!
AlexLos Angeles, CA
Thank you for all your help. It is nice to know that this service is here for people like myself, who need answers fast and are not sure who to consult.
GPHesperia, CA
I couldn’t be more satisfied! This is the site I will always come to when I need a second opinion.
JustinKernersville, NC
Just let me say that this encounter has been entirely professional and most helpful. I liked that I could ask additional questions and get answered in a very short turn around.
EstherWoodstock, NY
Thank you so much for taking your time and knowledge to support my concerns. Not only did you answer my questions, you even took it a step further with replying with more pertinent information I needed to know.
RobinElkton, Maryland
He answered my question promptly and gave me accurate, detailed information. If all of your experts are half as good, you have a great thing going here.
DianeDallas, TX
Also Check: What Is Reverse Psychology In Relationships
Overview: Scl2 Electron And Molecular Geometry
According to the VSEPR theory, the SCl2 molecule ion possesses tetrahedral molecular geometry. Because the center atom, sulfur, has two S-Cl single bonds with the two chlorine atoms surrounding it. The Cl-S-Cl bond angle is 103 degrees in the tetrahedral SCl2 molecular geometry. The SCl2 molecule has a tetrahedral geometry shape because it contains two chlorine atoms in the tetrahedral and two corners with two lone pairs of electrons.
There are two S-Cl single bonds at the SCl2 molecular geometry. After linking the two chlorine atoms and two lone pairs of electrons on the sulfur atom in the tetrahedral form, it maintains the tetrahedral-shaped structure. In the SCl2 molecular geometry, the S-Cl single bonds have stayed in the two terminals and two lone pairs of electrons on the sulfur atom of the tetrahedral molecule.
The center sulfur atom of SCl2 has two lone pairs of electrons, resulting in tetrahedral SCl2 electron geometry. However, the molecular geometry of SCl2 looks tetrahedral or v-shaped and has two lone pairs of electrons on the sulfur of the SCl2 geometry. Itâs the SCl2 moleculeâs symmetrical geometry. As a result, the SCl2 molecule is polar.
What Is The Hybridization Of Scl2
To calculate the hybridization of SCl2 follows some simple steps.
Step 1: Determine the number of atoms attached to the central atom as we know the central atom is that atom that has the least electronegativity.
So, Sulfur has the least electronegativity therefore by looking at SCl2 we determined that only 2 atoms are attached to the central atom.
The number of attached atoms to sulfur is = 2
Step 2: Now find the lone pair of central atoms
As the Valence electron for sulfur is 6 and from 6 electrons it shares only 2 electrons to make bonding with chlorine atoms. So, we left with 4 electrons which make 2 lone pairs.
Step 3: This is the final step to determine the hybridization of SCl2.
Hybridization = Number of atoms attached + Number of lone pairs
= 2 + 2
= 4 which makes the hybridization of SCl2 is sp3.
Read Also: Geometry Wars 2 Smile Achievement
What Are The Molecular Geometry Or Shape Of Scl2
Molecular geometry is the way of arrangement of atoms in molecules. By looking at the lewis structure of SCl2 many assume that it has a linear shape but they are wrong Because it is one of the biggest limitations in Lewiss theory as it does not help to determine the accurate shape of molecules.
So, for overcoming this problem VSEPR theory comes to determine the molecular shape of chemical compounds.
The molecular geometry of SCl2 is bent. Because it contains two lone pairs on the central atom that tries to repel each other and bonded pairs of electrons around it, as a result, it pushes down the bonded atoms giving the bent geometry structure.
Also, there are asymmetric charges distributed around the central atom, due to this, the repulsion generated by these lone pair electrons on the central atom causes the SCl2 molecule to become a bent geometrical structure.
How To Draw Lewis Structure For Scl2
Famous scientist Gilbert. N. Lewis first introduce lewis structure in the year of 1916 through his well known journal The Atom and the Molecule. This structural representation has a great significance in not only structure or shape determination but also in the calculation of nonbonding, bonding electrons of any molecular species.
The following steps should be followed to draw the lewis structure of any molecule.
Applying the above rules, lewis dot structure is drawn .
The increasing order of this above repulsive factor is-
Lone pair-lone pair repulsion > Lone pair-bond pair repulsion > bond pair-bond pair repulsion.
In SCl2, sulfur has two lone pair and each of the fluorine has three lone pairs. Lone pairs of the sulfur atom will face repulsion with each other and with the bond pair electrons also. Thus, the actual structure is slightly deviated and it is shown bent structure with sp3 hybridization.
Shape of SCl2
Also Check: Prentice Hall Gold Geometry Chapter 6 Answers
Scl2 Lewis Structure Octet Rule
Through this rule, an atom will get the electron configuration like its nearest noble gas molecule.
The atom will have tendency to take part in any reaction until its octet will be filled up because valance shell electrons will only participate in reaction. Noble gases are very much less reactive as they have full filled electron configuration in their respective valance shell.
But octet rule is violated in SCl2 because both of the sulfur and chlorine are group three element and they cannot have eight electrons in their valance shell. They have s, p and d orbitals. S, p and d orbital have the capacity of having electron 2,6 and 10 respectively. Thus, any group three element can have eighteen electrons in their valance shell.
So, SCl2 is an exception of octet rule due to presence of group III element .
Scl2 Molecule Is Polar Or Non
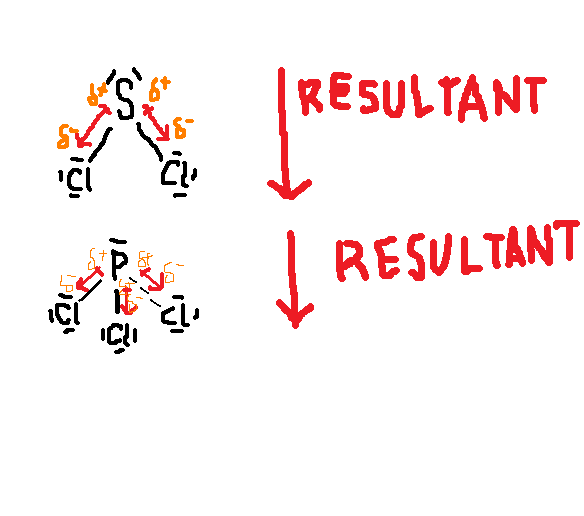
SCl2 molecule is polar because of its non-uniform charge distribution around the atoms and asymmetrical shape of the molecule. There are three major factors that can influence the polarity of the SCl2 molecule.
Electronegativity: Electronegativity of atoms is directly proportional to the polarity of molecules. The electronegativity of the chlorine atom is 3.16 and sulfur is 2.58 and the electronegativity difference between them is greater than 0.5 which indicates that the SCl2 molecule is polar in nature.
Dipole moment: Greater the dipole moment more is the polarity of the molecule. A higher electronegative atom attracts the bonded electron pair slightly more towards its side. As a result, a higher electronegativity atom gains a partial negative charge and another atom gains a partial positive charge.
This generates two poles across a molecule. Hence, dipole moment defines as the product of electric charge and distance between the positive and negative species found in the molecule. So, dipole moments arises only when differences in the electronegativity of molecules. The dipole moment is a measure of the polarity of the molecule.
Geometrical structure: The shape is also an important factor to determine whether the molecule is polar or non-polar. More the asymmetrical shape of molecules greater is the dipole moment whereas symmetrical structure dipole moment can easily be canceled out with each other and make non-polar in nature.
You May Like: What Do You See Pictures Psychology
What Is The Bent Molecular Geometry Of Scl2
SCl2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm. Start with the molecules Lewis structure, which is drawn like this: http://www.tutor-pages.com/Chemistry/Molecular_Geometry/035_Sulfur_Dichloride_SCl2.html It is important to remember
What is the molecular shape of S c l2?
What is the molecular shape of S C l2? SCl2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm. Start with the molecules Lewis structure, which is drawn like this:
Is the bond length of SCl2 higher than H2O?
SCl2 molecular geometry is very similar to H2O but its bond angle is slightly lower than H2O because of the lone pair. As higher the lone pair, the smaller is the bond angle and the bond length of SCl2 is also higher than H2O. What is the Hybridization of SCl2? To calculate the hybridization of SCl2 follows some simple steps.
Calculating Lone Pair Of Electrons On Chlorine In The Scl2 Geometry:
Finding lone pair of electrons for the terminal atom is not similar to the central sulfur atom. We use the following formula as given below
Use the formula below to find the lone pair on the Chlorine atom of the SCl2 molecule.
L.P = V.E â N.A
Lone pair on the terminal Chlorine atom in SCl2 = L.P
Terminal Chlorine atomâs valence electron in SCl2 = V.E
Number of S-Cl bonds = N.A
calculation for Chlorine atom lone pair in SCl2 molecule.
For instance of SCl2, their terminal atoms, Chlorine, have seven electrons in its outermost valence shell, one S-Cl single bond connection. This gives a total of two S-Cl single bond connections. But we are considering only one connection for the calculation.
As a result of this, L.P = =6
The lone pair of electrons in the Chlorine atom of the SCl2 molecule is six. Two Chlorine atoms are connected with the central sulfur atom.
In the SCl2 electron geometry structure, the lone pairs on the central sulfur atom are two, lone pairs of electrons in the Chlorine atom have six. Two Chlorine atoms have 12 lone pairs of electrons.
It means there are two lone pairs of electrons in the core sulfur atom. Two lone pair of electrons on the central sulfur atom is responsible for the tetrahedral nature of SCl2 molecular geometry. But in the structure Chlorine atoms are polarised sidewise in their tetrahedral geometry.
Don’t Miss: Chapter 12 Test Form 2b Geometry Answers
Scl2 Molecular Geometry Or Shape
The bond angle of SCl2 is approx 103º.
The electron pair geometry of SCl2 is tetrahedral as it considers lone pair on the central atom as well as bonded pair around it.
The generic formula for SCl2 is AX2N2 according to the VSEPR theory and its the chart.
SCl2 molecular geometry is very similar to H2O but its bond angle is slightly lower than H2O because of the lone pair. As higher the lone pair, the smaller is the bond angle and the bond length of SCl2 is also higher than H2O.
Molecular Geometry And Shape Of Scl2
Molecular geometry simply represents the molecular structure of the molecule. It is a three-dimensional representation of a molecule that provides actual arrangements of atoms in a molecule. Most of the properties of a molecule are determined by its geometry.
We can determine SCl2 molecular geometry and shape according to VSEPR theory. VSEPR theory predicts the shape of the molecules depending on the no. of bond pair and lone pair of electrons around the central atoms. For that, we have to use the AXN method.
AXN notation for SCl2 molecule:
A is the representation for the central atom, so, in the SCl2 molecule, S is the central atom which means A = sulfur
X represents the number of bonded atoms with the central atom, i.e., sulfur is bonded with two atoms . Therefore, X = 2
N represents the total number of lone pairs of electrons present on the central atom. There is two lone pair of electrons present on the central sulfur atom so that N = 2
Now the generic AXN formula for the SCl2 molecule becomes AX2N2.
According to VSEPR theory, if the molecule has a generic formula AX2N2 then its molecular geometry bent and electron geometry will be tetrahedral.
Don’t Miss: What Does Cyte Mean In Biology