Summary Critical Point Vs Triple Point
Critical point of a substance is the end point of the phase equilibrium curve of that substance which gives the temperature and pressure at which liquid and vapour phase of a substance can coexist with each other. The triple point gives the temperature and pressure at which all three phases of matter can coexist with each other. The difference between a critical point and the triple point is that critical point describes the coexistence of two phases of same substance whereas triple point describes the coexistence of three phases of the same substance.
Reference:
1.Helmenstine, Anne Marie, D. Triple Point Definition and Example . ThoughtCo, Nov. 10, 2017. Available here2.Triple point. Wikipedia, Wikimedia Foundation, 6 Mar. 2018. Available here 3.Critical Point. Chemistry LibreTexts, Libretexts, 21 July 2016. Available here
Image Courtesy:
1.Phase-diag2 By Matthieumarechal, via Commons Wikimedia
Triple Point Of Water
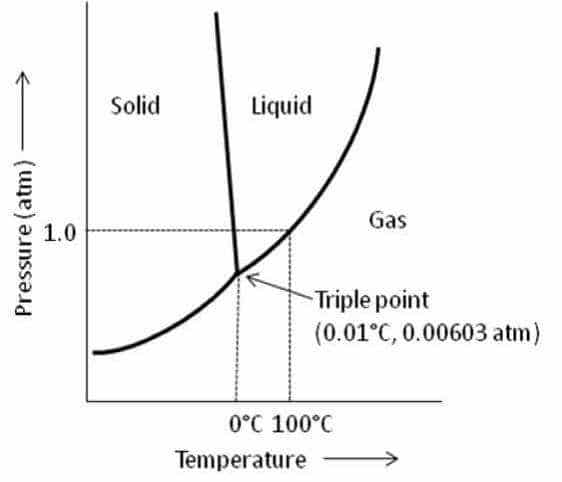
The single combination of pressure and temperature at which pure water, pure ice, and pure water vapour can coexist in a stable equilibrium occurs at exactly 273.16 K and a pressure of 611.73 pascals . At that point, it is possible to change all of the substance to ice, water, or vapor by making infinitely small changes in pressure and temperature. Strictly speaking, the surfaces separating the different phases should also be perfectly flat, to avoid the effects of surface tensions.
Water has an unusual and complex phase diagram, although this does not affect general comments about the triple point. At high temperatures, increasing pressure results first in liquid and then solid water. At lower temperatures under compression, the liquid state ceases to appear, and water passes directly from gas to solid.
At constant pressures above the triple point, heating ice causes it to pass from solid to liquid to gas, or steam, also known as water vapor. At pressures below the triple point, such as those that occur in outer space, where the pressure is near zero, liquid water cannot exist. In a process known as sublimation, ice skips the liquid stage and becomes steam when heated.
What Customers Are Saying:
Wonderful service, prompt, efficient, and accurate. Couldn’t have asked for more. I cannot thank you enough for your help.
Mary C.Freshfield, Liverpool, UK
This expert is wonderful. They truly know what they are talking about, and they actually care about you. They really helped put my nerves at ease. Thank you so much!!!!
AlexLos Angeles, CA
Thank you for all your help. It is nice to know that this service is here for people like myself, who need answers fast and are not sure who to consult.
GPHesperia, CA
I couldn’t be more satisfied! This is the site I will always come to when I need a second opinion.
JustinKernersville, NC
Just let me say that this encounter has been entirely professional and most helpful. I liked that I could ask additional questions and get answered in a very short turn around.
EstherWoodstock, NY
Thank you so much for taking your time and knowledge to support my concerns. Not only did you answer my questions, you even took it a step further with replying with more pertinent information I needed to know.
RobinElkton, Maryland
He answered my question promptly and gave me accurate, detailed information. If all of your experts are half as good, you have a great thing going here.
DianeDallas, TX
Recommended Reading: Is Physics Required In High School
Entropy And Its Properties
- Entropy is a function of thermodynamics.
- It is a state function. Instead of the path chosen, the outcome is determined by the systems condition.
- It is generally represented by S however, in the normal state, it is represented by S°.
- J/Kmol is the SI unit.
- Cal/Kmol is the CGS unit.
- The entropy of a system is an extensive quality, meaning it expands in proportion to its size or scope.
An isolated system will have more disorder, leading to a greater entropy. Entropy also increases when chemical reactions break down into more products. In a system at a higher temperature, randomness is greater than at a lower temperature. With these examples, it is clear that entropy increases with a decrease in regularity.
Discover And Learn What Students Are Asking
Also Check: What Is Interference In Physics
Solution For Problem 132 Chapter 12
General Chemistry: Principles and Modern Applications | 10th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
General Chemistry: Principles and Modern Applications | 10th Edition
If the triple point pressure of a substance is greater than 1 atm, which two of the following conclusions are valid? The solid and liquid states of the substance cannot coexist at equilibrium. The melting point and boiling point of the substance are identical. The liquid state of the substance cannot exist. The liquid state cannot be maintained in a beaker open to air at 1 atm pressure. The melting point of the solid must be greater than The gaseous state at 1 atm pressure cannot be condensed to the solid at the triple point temperature.
Textbook: General Chemistry: Principles and Modern Applications
Edition: 10
ISBN: 9780132064521
Other solutions
Entropy Constant At The Triple Point Of Water
The term triple point refers to a condition in which the solid, liquid, and gas phases are all in equilibrium simultaneously. Salient features of entropy are discussed in the article.
Table of Content
The entropy of a system refers to the amount of thermal energy per unit temperature that cannot be used. Molecular motion results in work thus, entropy is also a measure of the disorder or randomness of a system. It calculates the energy an object is not able to use to do work. It also measures the number of arrangements that an atom can take in a system. According to the Second Law of Thermodynamics, entropy remains the same regardless of the direction of time. The entropy can only be constant if the system is in the most disorderly state possible. At the triple point, three phases of a particular substance can coexist simultaneously, such as gaseous, liquid, and solid. Therefore, the entropy is constant at the triple point of water. The direction of spontaneous change based on entropy can explain several phenomena. The German physicist Rudolf Clausius introduced a key element of 19th-century physics in 1850.
Recommended Reading: What Does Dsm Stand For In Psychology
What Is The Triple Point Pressure For Gallium
I have seen various discussions about the triple point of Gallium determined to a very precise value, so precise that it is used as a reference for NIST scales and measurements.
However, these reference related documents mean ‘temperature’ only when talking about triple point. Triple-point is defined as a pair, so I fail to understand why despite so many discussions on the triple point of Ga, the pressure value is not easy to find.
I did find a value of $10^ \mathrm$ sometime in the past but I can’t find the source. However I have two questions related to this value:
- Does it even makes sense to talk about such a minuscule value of pressure? Wouldn’t this mean one particle every cubic light-year or something? .
- Is it safe to say that Gallium melts at absolute zero pressure , and never sublimates or desorbs ?
In general, are there other metals that have such a small triple-point pressure that they are guaranteed to never exhibit the process of sublimation/desorption, even in a perfect vacuum? .
- 1$\begingroup$@JonCuster Thanks. Very helpful resource. I calculated the pressure using eq. 8 and temperature of $302.9166$ K. I get $6.77 \times 10^$ atm. Since this is pretty much as minuscule as $10^$ atm, posted questions are still relevant.$\endgroup$
What Is Triple Point
The Triple is considered as a thermodynamic equillibrium point. At this point all the three states of an element Solid, Liquid & Gas form considered at an equillibrium. Triple point is different for different system.
Example, Triple point of Acetylene occurs at -80.7° Celsius.
Triple point of any substance is a single point at a peculiar temperature and pressure. It is the point where all the three phases coexists at equillibrium.
For Example : Consider water as a system. The triple point of water is at 273.16 K and the assosiated pressure is 611.2 Pa.
Similarly, For Carbon Dioxide the triple point coexist at 5.2 atm and 216.6 K .
Read Also :
Don’t Miss: What Is The Physics Primer
Types Of Hydrogen Bonding
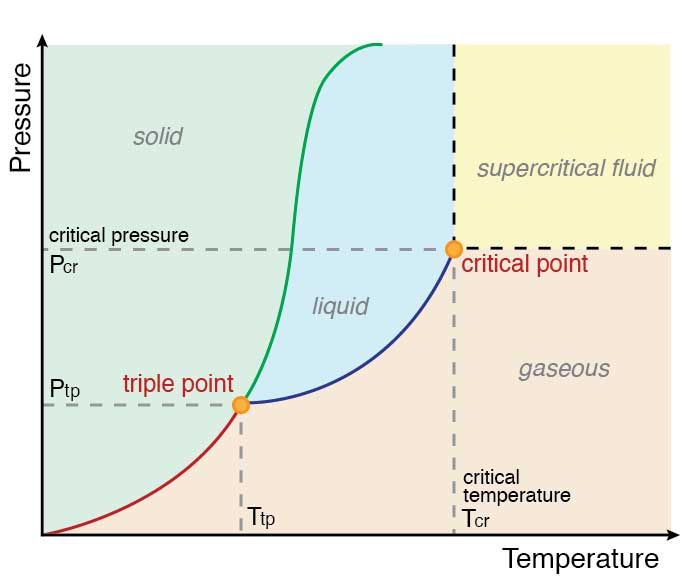
Critical Point And Triple Point
Critical Point and Triple Point are terms which refer to a certain state a substance. These terms are related with equilibrium between phases. Phase diagrams are charts which convey information of different states of a material Solid, Liquid, Gas and presence of equilibrium or non-equilibrium conditions between them at different Temperatures, Pressures and Volume.
Critical point and triple point can be located on a phase diagram. Phase diagrams having variables other than the common variables of temperature, pressure and volume such as enthalpy, entropy etc are also used in industries to understand or design feasible systems for turbines, compressors, air conditioners etc. Each substance has a unique phase diagram.
The above phase diagram is called a pressure-temperature phase diagram because the chart uses pressure and temperature as its variables. The lines on the diagram represent the conditions of temperature and pressure where at least two phases of the substance co-exist in equilibrium with each other.
At least two phases means it can be solid or liquid, liquid or gas, gas or solid, or all the three phases together. If the pressure and temperature of the material is such that the points dont lie on the lines then the substance will exist in only one single phase, either as a solid or liquid or a gas.
The critical point and triple point are labelled on the diagram. Both points act as end points of the vaporisation curve.
You May Like: What Is Grid In Geography
Naming Alkenes And Alkynes
Learn What The Triple Point Means In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in equilibrium. It is a specific case of thermodynamic phase equilibrium. The term “triple point” was coined by James Thomson in 1873.
Don’t Miss: How To Teach Kindergarten Math Skills
Change In Entropy And Its Calculations
As part of an entropy change, a process is defined as the amount of heat emitted or absorbed isothermally and reversibly divided by the absolute temperature. The entropy formula is as follows:
S = qrev,iso/T.
When the same quantity of heat is added at higher and lower temperatures, randomness will be highest at the lower temperature. It follows that temperature is inversely proportional to entropy.
- Thermodynamically, spontaneous processes are irreversible.
- After some time, the irreversible process will reach equilibrium.
Total entropy change, Total = Surroundings + System
The total entropy change is equal to the sum of the entropy changes in the system and its surroundings.
If a system loses heat q at a temperature T1, which is received by surroundings at a temperature T2, S total can be calculated as,
System = -q/T1
- When Total is positive, the process is spontaneous.
- If Total is negative, then the process is non-spontaneous.
- When Total is zero, the process is in equilibrium.
When an ideal gas expands isothermally reversibly, the entropy changes
S = qrev,iso/T.
Following the first law of thermodynamics,
U = q + w
The isothermal expansion of an ideal gas is, U = 0
qrev = -wrev = nRTln
S = nRln
Key Difference Critical Point Vs Triple Point
Critical point and triple point are terms used to explain temperatures and pressures at which two or more phases of substances can coexist with each other. The critical point is the condition at which the liquid and vapour phase of the same substance coexist. The triple point is the condition at which all three phases of matter can coexist with each other. The key difference between a critical point and the triple point is that critical point describes the coexistence of two phases of same substance whereas triple point describes the coexistence of three phases of the same substance.
Also Check: How To Calculate Heat Change In Chemistry
Effects Of Hydrogen Bonding On Elements:
Association: The molecules of carboxylic acids exist as dimer because of the hydrogen bonding. The molecular masses of such compounds are found to be double than those calculated from their simple formula.
Dissociation: In aqueous solution, HF dissociates and gives the difluoride ion instead of fluoride ion. This is due to hydrogen bonding in HF. The molecules of HCl, HBr, HI do not form a hydrogen bond. This explains the non-existence of compounds like KHCl2, KHBr2, KHI2.
What Is Critical Point
The critical point of a substance is the end point of the phase equilibrium curve of that substance. A phase equilibrium curve or a phase diagram is the graph of pressure versus temperature in which the phase changes of the substance is shown. This shows the temperatures and pressure at which the substance exists as a solid, liquid or gas. The critical point is the temperature and pressure at which the liquid and vapour phase coexist.
Figure 01: A Phase Diagram showing Both Critical Point and Triple Point
The temperature and pressure at critical point are named as critical temperature and critical pressure . As shown in the above image, the lines between two phases are known as boundaries. A critical point indicates the point at which line boundaries vanish.
Knowing the critical point of a substance is sometimes very important. For example, a gas can never be condensed at temperatures and pressures above its critical point. This is because the intermolecular forces between gas molecule are weakened at very high temperatures since the kinetic energy of those molecules is increased.
There are two types of the critical point
Also Check: What Is Natural Selection In Psychology
Moving About The Diagram
Moving about the phase diagram reveals information about the phases of matter. Moving along a constant temperature line reveals relative densities of the phases. When moving from the bottom of the diagram to the top, the relative density increases. Moving along a constant pressure line reveals relative energies of the phases. When moving from the left of the diagram to the right, the relative energies increases.
Phase Changes And Equilibrium
- In liquid: Representative values of phase-diagram parameters
the particular values of the triple-point and critical-point temperature and pressure, the size of the various regions, and the slopes of the lines. Triple-point temperatures range from 14 K , for hydrogen to temperatures too high for accurate measurement. Triple-point pressures are generally low,
Read Also: How To Calculate Average Uncertainty In Physics
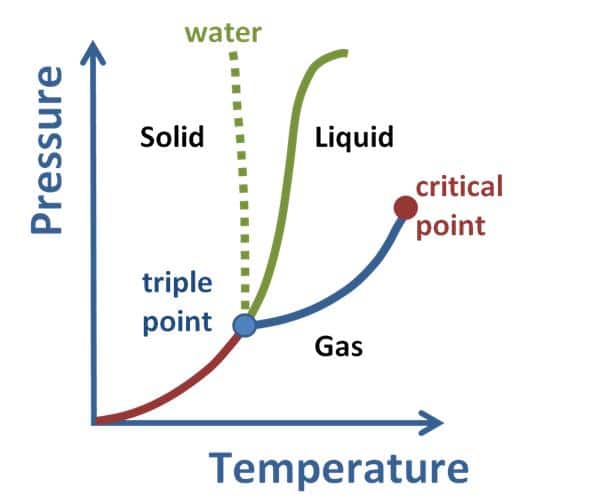
Critical Point Vs Triple Point
The critical point of a substance is the end point of the phase equilibrium curve of that substance. The triple point is the temperature and pressure at which solid, liquid, and vapour phases of a particular substance coexist in equilibrium. Phases The critical point is the end point of a phase diagram curve. Triple point is the point at which all the boundary line meet with each other.
Applications Of Multiple Integrals
You May Like: What Does Competition Mean In Biology
What Are The Triple Point Pressure And Temperature For Pure Cyclohexane
Here is a video that demonstrates the interesting nature of the triple point of cyclohexane, so I tried researching on the internet for a phase diagram, but only found data from a manufacture where the triple point was specified as 279.48 degK at 5.388 kPa. But in the video there doesn’t seem to be a cooling plate. The flask appears to be at room temperature.
What are the triple point specs for pure cyclohexane? If they match the data above, how were they able to achieve the phase transitions shown in the video at room temperature?
Also where can I find a phase diagram for pure cyclohexane on the internet?
- $\begingroup$I suspect that with volatile solvents like cyclohexane, the vacuum pump you hear in that video can do a good job of cooling the flask via evaporative cooling.$\endgroup$
The NIST REFPROP program reports the triple-point temperature of cyclohexane as 279.47 K.The program refers to: S. G. Penoncello, R. T. Jacobsen, and A. R. H. Goodwin: A Thermodynamic Property Formulation for Cyclohexane, International Journal of Thermophysics, Vol. 16., No. 2, 1995, 515531.This publication mentions the triple-point temperature as T = 279.47 K and the triple-point pressure as p = 5.388 kPa.The given reference is: J. G. Aston, G. J. Szasz, and H. L. Fink, J. Am. Chem. Soc.65 1135 .