Who Is Responsible For Using Quality Management System
According to current Good Manufacturing Practice , medical device manufacturers have the responsibility to use good judgment when developing their quality system and apply those sections of the FDA Quality System Regulation that are applicable to their specific products and operations, in Part 820 of the QS
What Is Kc In Chemistry
The equilibrium constant Kc in chemistry or Keq indicates the ratio of the concentration of all substances involved in a chemical equilibrium reaction. It is used in particular in connection with the law of mass action. That is why it is often referred to as the mass action constant, more rarely as the equilibrium constant. In contrast to the position of the equilibrium, the value of the equilibrium constant depends only on the temperature and not on the concentration or the pressure.
For any reversible reaction at equilibrium, the ratio of products to reactants at a certain temperature will always equal a constant, Kc
In general,
Kc= Concentration of products/ Concentration of reactants
Specifically, for any reversible reaction at equilibrium
aA + bB cC + dD
The equilibrium constant, Keq Or KC, is
Kc = c d / a b
College Of American Pathologists Laboratory Accreditation Program Requirements For Quality Control
CAP-LAP’s philosophy is that all clinical laboratory tests need to follow the requirements defined for high complexity testing under CLIA’88 . Requirements for routine analysis of QC follow the CLIA requirements in terms of number and frequency, except controls must be included with all tests . For now, CAP only allows “acceptable” alternative QC for point of care testing. The latest POCT Checklist states that certain unit-use devices/kits may warrant some combination of instrument, procedural, and/or electronic controls. Question 30.0550 states that POCT sites using alternative QC must have scientifically acceptable evidence that the entire analytical process is being evaluated correctly. Except for electronic controls, CAP requires that control specimens be tested in the same manner as patient samples.
Qualitative tests need to be evaluated with both a positive and negative control each day of use. For all tests, CAP-LAP requires an audit trail that ties the patients results with the analyst, instrument and QC. In addition, the QC program should show evidence of documented review on the next shift, if no supervisor is on site, and at least weekly review by the technical supervisor and monthly secondary review by the director or director’s designee.
Recommended Reading: Definition Of Abiotic Environment
How Do You Know If A Reaction Is Reversible
In a reversible reaction, both forward and reverse directions of the reaction generally occur at the same time. While reactants are reacting to produce products, products are reacting to produce reactants. Often, a point is reached at which forward and reverse directions of the reaction occur at the same rate.
What Is Required For A Reaction To Reach Equilibrium
The reaction must be reversible, and there must be a closed system. A chemical reaction doesnt stop when equilibrium has reached. Instead, the rate of forward reaction equals the rate of reverse reaction. A dynamic equilibrium exists once the concentrations of reactants and products becomes constant.
Recommended Reading: Glencoe Algebra 1 Chapter 7 Test Form 2a Answers
The Quality Control Procedure
In order to implement an effective QC program, an enterprise must first decide which specific standards the product or service must meet. Then the extent of QC actions must be determined — for example, the percentage of units to be tested from each lot.
Next, real-world data must be collected — such as the percentage of units that fail — and the results reported to management personnel. After this, corrective action must be decided upon and taken. For example, defective units must be repaired or rejected, and poor service repeated at no charge until the customer is satisfied. If too many unit failures or instances of poor service occur, a plan must be devised to improve the production or service process then that plan must be put into action.
Finally, the QC process must be ongoing to ensure that remedial efforts, if required, have produced satisfactory results and to immediately detect recurrences or new instances of trouble.
Continue Reading About quality control
Biography: Sharon S Ehrmeyer Phd
Sharon Ehrmeyer, Ph.D., MT is Professor of Pathology and Laboratory Medicine and Director of the Medical Technology Program at the University of Wisconsin in Madison, Wisconsin. Dr. Ehrmeyer is active in the American Association for Clinical Chemistry, the American Society for Clinical Laboratory Science and the National Committee Clinical Laboratory Standards where she serves on the Board of Directors and chairs its pH/Blood Gas Committee. Dr. Ehrmeyer gives numerous presentations on laboratory regulations , point of care testing and various quality issues. Her research interests focus on clinical laboratory quality and the impact of government regulations on laboratory practices.
Also Check: Jonathan Thomas Beth Thomas Brother Now
What The Heck Is Qc In Chemistry And What Exactly Is Qc About
What is qc in chemistry and what on earth is on about? Allow us start out aided by the definition of Q. Q could possibly be your velocity of petrol, as a operate of temperature, strain, quantity, or community.
By definition of Qwe know that Q is in a aqueous location wherever its the warmth of response of the petrol. All compound reactions have got to shift by using this period of petrol and heating.
Considering that the molecules of the petrol are shifting by way of the gadget Heating vitality is converted into the gas. Speeds plus the moves of the molecules modification and there exists a adjust in the cost of combustion or fuel development, even while it happens to be however a fuel.
It happens to be significant to remember there are. Chemical reactions just take position. The elements utilized to do the reactions impact the fluid and gasoline houses.
Really good chemistrys a few components have already been chemistry, situations, and dimension. Chemistry will contain education of qualities of these fluids, gases, solids, and metals of these compounds, prerequisites so you can get their separation or mixing, and measurement techniques and methods for detecting the reaction and its response choices.
Whats qc in chemistry in addition to whats about? It is really the pace of gasoline.
Clia Qc Requirements Based On Test Complexity
CLIA’88 regulations are based on four categories of test complexity: waived, provider performed microscopy , moderate complexity, and high complexity. Current information on test complexity can be obtained from the CDCs web site . Each testing category has different regulatory requirements for personnel, quality control, quality assurance, proficiency testing, etc.
Read Also: Kuta Software Surface Area Of Pyramids And Cones
What Is Quality Assurance In Clinical Chemistry
quality assuranceclinical chemistrycontrolcontrol
. Also to know is, what is quality assurance in chemistry?
Quality assurance and quality control are two of the main activities that are required to ensure a quality product. Chemists who work in quality assurance enjoy finding ways to reduce the possibility of error in manufacturing methods by using a big-picture view of product quality.
Also Know, what is quality control in clinical chemistry? Laboratory quality control is designed to detect, reduce, and correct deficiencies in a laboratory’s internal analytical process prior to the release of patient results, in order to improve the quality of the results reported by the laboratory.
Also to know is, what is quality assurance in clinical laboratory?
Quality assurance is aimed at ensuring quality test results. The purpose of quality assurance is to give relevant, reliable, timely test results which is interpreted correctly. Quality assurance involves activities both inside and outside laboratory, good laboratory practice and proper management skilll.
What is the importance of quality control and quality assurance in the medical laboratory?
The importance of quality control in laboratory testing. Quality control refers to the process of detecting analytical errors within the lab to ensure both the reliability and accuracy of test results in order to provide the best possible patient care.
What The Heck Is Qc In Chemistry And What Is Qc About
What specifically is what is actually about and qc in chemistry? Let us start out with all the definition of Q. Q may be the velocity of petrol, staying a operate of temperature.
Warmth vitality has become switched into the petrol for the reason that atoms of these gasoline are all moving thru the device. Fees additionally, the moves of the molecules change and a modification is in the rate of combustion or gasoline development, even although it can be however a gasoline.
Its essential to note that there are some reasons that could impact these adjustments. Chemical reactions take site. The substances utilized to do the reactions impact fluid belongings as well as gas.
Incredibly good chemistrys a few elements seem to have been states, all chemistry, and measurement. Chemistry entails conditions which include their separation or mixing awareness of all factors of these gases, liquids, solids, and alloys of these substances, and dimension techniques and tools which includes detecting the response and its response items.
What exactly is on about qc in chemistry and also What is? They have been referring to chemistry when people today in the mathematics community discuss about this this affiliation amongst the resources, the gasoline, and the gas combinations which might be designed and analyzed.
Exactly what is qc in chemistry and what is actually about about? It happens to be the pace of fuel.
Read Also: Define Surface Area In Math
Student Health Center Manuals
PURPOSE
To identify control materials used throughout the laboratory and to formulate a comprehensive plan to perform, monitor, evaluate and take corrective action with control values obtained. A comprehensive quality control plan allows laboratory personnel to record and monitor the daily performance of the analyzers and reagents used throughout the laboratory and to predict shifts and trends in control materials over a period of time.
Expiration date checking is a component of Laboratory Quality Control, and all products with expiration dates are checked monthly, with any expired or expiring products sequestered and removed from use.
PROCEDURE FOR PERFORMANCE OF CONTROL MATERIAL
ABAXIS PICCOLO
The Abaxis Piccolo is a Chemistry test system that incorporates internal quality control mechanisms that monitor all potential sources of error throughout the testing process. The Piccolo selfcalibrates with each patient run, utilizing the onboard, continuous intelligent Quality Control , which monitors the analyzer, reagent reactions and sample to ensure chemistry and instrument integrity. As a waived test system external quality controls are run after each shipment, each different lot # or every 30 days, whichever comes first.
It is also important to check QC in the following situations:
a. Whenever laboratory conditions have changed significantly
b. When training or retraining of personnel is indicated
c. When test results do not match patient symptoms or clinical findings
What Is Actually Qc In Chemistry And What Precisely Is Qc About

So what particularly is qc in chemistry and exactly what is on about? Permit us begin when using the definition of Q. Q may just be that the velocity of petrol, as a perform of temperature, gurudissertation.net/ stress and anxiety, quantity, or room.
Warmth electrical power is transformed to the petrol for the reason that atoms of the petrol are transferring by means of the unit. Speeds together with the motions of these molecules transform and you can find a big modify at the cost of combustion or gasoline formation, while it is actually nonetheless an gas.
It is actually vitally important to be aware there are some items that can impact these adjustments. Chemical reactions acquire position at quite a lot of pressures and temperatures. The resources used to execute the reactions guidance figure out the fuel and fluid belongings.
The 3 parts of chemistry are states, all chemistry, and measurement. Chemistry needs comprehension of attributes of their gases, liquids, solids, and alloys of the compounds, disorders similar to their mixing or separation, and in addition dimension approaches and products that include finding the response and its very own reaction solutions.
What is actually about qc in chemistry and likewise What is actually? When men and women at the arithmetic industry speak they have been referring to chemistry.
Recommended
You May Like: Holt Mcdougal Geometry: Practice And Problem Solving Workbook Answer Key
The General Role Of Qa/qc
In order to understand the scope of what QA/QC does for the chemical and petrochemicalsector specifically, it is first necessary to understand the general role QA/QC plays forcompanies regardless of the industry. Whether the company in question manufactures or workswith chemical substances or makes office supplies or electronics, the general role of QA/QC isas follows:
Quality Assurance Quality assurance involves creating and optimizing systems andprocesses that assure peak quality. The aim of quality assurance is to prevent a problem ordefect before it occurs by establishing a framework that yields consistently high quality results.
Quality Control By contrast quality control acknowledges the reality that some errors,problems, or defects are going to slip through no matter what, but that catching and resolvingthese issues is critical. A major part of quality control is thus testing and analyzing finishedproducts to detect and correct any sub-quality results.
Jcaho And Moderate Complexity Tests
For moderate complexity tests, JCAHO, for the most part, follows CLIA’88 and mandates the same seven QC requirements as CLIA88 and COLA and also accepts electronic controls for now. However, for use of electronic controls, JCAHO requires that the laboratory verify the manufacturers QC claims and run external controls periodically to validate that no change occurred with the testing system.
You May Like: How Many Biological Children Does Nicole Kidman Have
Jcaho And Waived Testing
JCAHO recognizes waived tests as defined by CLIA’88, but identifies additional QC requirements that include:
- Defined QC checks that at least meet the minimum manufacturer’s recommendations
- Maintenance of appropriate QC and test records
- Proof of training and continued competence of all testing personnel
- Proof all testing personnel have access to current written procedures for QC and remedial actions.
- Maintenance of QC records, including a mechanism to correlate or link analyst, QC records, instrument and instrument problems, and individual patient test results.
- For glucose, at a minimum two levels of controls are required each day a glucose is performed. QC is focused on each meter used, not each individual performing the test. Therefore, not all testing personnel need to routinely perform QC, but each meter must be validated by QC before testing patient samples.
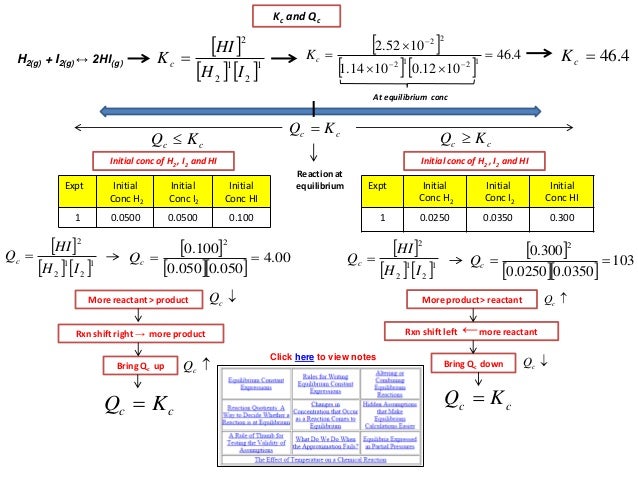
What Condition Must Be Satisfied So That Qc Kc
Any given chemical reaction at equilibrium has an equilibrium constant, K, associated with it. We do not need any shift in the reaction to reach equilibrium for Qc = Kc since it already means that the reaction is at equilibrium. Therefore: Qc = Kc is satisfied when the reaction is at equilibrium condition.
Read Also: My Hrw Com Algebra 1
What Exactly Is Qc In Chemistry And What Is Qc About
Subsequently what is qc in chemistry and exactly what is about? Let us commence together with the definition of Q. Q is the velocity of gasoline, staying a use of temperature, pressure, quantity, or room.
Heating power is converted to the petrol considering the molecules of the gas are going as a result of the equipment. Speeds along with the moves of the molecules modification and you can find a transform in the rate of combustion or fuel formation, though it really is even now a gas.
It really is extremely important to bear in mind there are. Chemical reactions acquire location. The substances employed to execute the reactions affect fluid properties along with the fuel.
The 3 facets of chemistry that is exceptional have been completely ailments, all chemistry, and also measurement. Chemistry will require comprehension of components of their gases, liquids, solids, and metals of these substances, conditions to obtain their mixing or separation, in addition to dimension strategies and applications along the lines of getting the response as well as its reaction programs.
What is about qc in chemistry and what the heck is? When individuals at the tech trade converse about this connection among the product, your gas, and in addition the petrol mixtures that can be built and examined, they are really referring to chemistry.
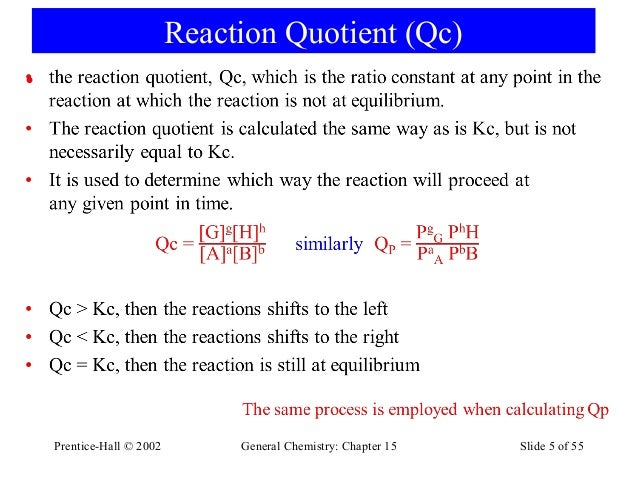
When Qc Kc A Reaction Will
Q can be used to determine which direction a reaction will shift to reach equilibrium. If K > Q, a reaction will proceed forward, converting reactants into products. If K reaction will proceed in the reverse direction, converting products into reactants. If Q = K then the system is already at equilibrium.
Recommended Reading: Elton John Children Biological
Difference Between K And Q
Sometimes it is necessary to determine in which direction a reaction will progress based on initial activities or concentrations. In these situations, the relationship between the reaction quotient, \, and the equilibrium constant, \, is essential in solving for the net change. With this relationship, the direction in which a reaction will shift to achieve chemical equilibrium, whether to the left or the right, can be easily calculated.
Jcaho Standards For Quality Control
In addition to the requirements identified above, JCAHO requires that all testing sites meet the following standards associated with quality control.
General QC Requirements:
- Each specialty and subspecialty has a documented quality control program.
- The laboratorys QC system includes daily surveillance of results by appropriate personnel.
- The laboratory takes remedial action for deficiencies identified through QC measures or authorized inspections and documents such actions.
- The laboratory ensures that QC results meet its criteria for acceptability before it reports patient test results.
Clinical Chemistry QC Requirements:
- Using appropriate controls, the laboratory verifies each procedure in clinical chemistry at least once each day of use.
- Using repetitive testing, the laboratory establishes control ranges with valid statistical measurements for each procedure in chemistry.
- The laboratory has established and makes available to its staff acceptable limits for all standard and reference QC samples, as well as the action to take when results are outside satisfactory control limits.
- The laboratory established test control limits to provide results with meaningful clinical applications.
Hematology and Coagulation QC Requirements:
Recommended Reading: Volume Formula Physics