S Of Measuring Osmotic Pressure
The osmotic pressure is determined by the following methods
- Pfeffers method
- Morse and Frazers Method
- Plasmolysis Method
- Townsends porous disc method
- Berkley and Hartleys Method
The first 5 methods take longer to measure osmotic pressure and there is also a possibility of a semi permeable membrane bursting. In 1909, berkley and hartley presented the most appropriate method, removing both these shortcomings. Which is described below
List Of Some Examples Of Osmosis
The real-life examples of osmosis are:
What Is Osmotic Pressure
Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane . It is a colligative property and is dependent on the concentration of solute particles in the solution. Osmotic pressure can be calculated with the help of the following formula:
= iCRT
- C is the molar concentration of the solute in the solution
- R is the universal gas constant
- T is the temperature
This relationship between the osmotic pressure of a solution and the molar concentration of its solute was put forward by the Dutch chemist Jacobus vant Hoff. It is important to note that this equation only holds true for solutions that behave like ideal solutions.
Also Check: How Many Subfields Of Psychology Are There
Osmotic Pressure And Food Preservation
The drying of fruit, the use of sugar to preserve jams and jellies, and the use of salt to preserve certain meats, are age-old methods of preserving food. The idea is to reduce the water concentration to a level below that in living organisms. Any bacterial cell that wanders into such a medium will have water osmotically drawn out of it, and will die of dehydration. A similar effect is noticed by anyone who holds a hard sugar candy against the inner wall of the mouth for an extended time the affected surface becomes dehydrated and noticeably rough when touched by the tongue.
In the food industry, what is known as wateractivity is measured on a scale of 0 to 1, where 0 indicates no water and 1 indicates all water. Food spoilage micro-organisms, in general, are inhibited in food where the water activity is below 0.6. However, if the pH of the food is less than 4.6, micro-organisms are inhibited (but not immediately killed] when the water activity is below 0.85.
Understanding The Osmotic Pressure Formula
$$\Pi = iMRT$$
Can this be rewritten as $\Pi = ORT$ where $O =$ osmolarity? The only reason I ask is because my book doesnt talk about the relationship between osmolarity and molarity, but I came across a practice problem where I had to find the minimum pressure required to purify sea water given the osmolarity of the seawater and temperature .
Also, this practice problem got me thinking about the relationship between osmolarity and molarity. Without knowing what the compound is in the seawater, theres no way to determine the molarity, correct?
A compound like glucose would have a vant Hoff factor of 1 so its molarity would be equal in value to the osmolarity. However, a compound like $\ce$ would have a molarity equal to half the osmolarity.
Here is the word problem for context:
Reverse osmosis is a process that allows fresh water to be obtained by using pressure to force an impure water source through a semi-permeable membrane that only allows water molecules to pass. What is the minimum pressure that would be required to purify seawater at $\pu$ that has a total osmolarity of $\pu$?
I dont want an answer to this problem because I have the answer and I know how to calculate it. But if it helps to answer the general question, please feel free to use the problem as a framework for a more general answer.
Read Also: Who Are The Biological Parents Of Prince Paris And Blanket
Berkley And Hartley Method
In this method a thin membrane of copper ferrocyanide prepared by the method is applied in the holes of a porous pot. This membrane acts as a semi-permeable membrane.
The porous pot fills this vessel in a cylindrical vessel, which is made of metal, by filling it with pure water. The solution whose osmotic pressure has to be found, fills it in this cylindrical vessel, there is a piston and pressure measuring device.
The porous pot has a tube in which the floor of water is noted before starting the experiment. When osmosis starts, the water starts going through the membrane towards the solution, which causes the water level in the tube to fall downwards.
Now with the help of piston, put so much pressure on the solution that the bottom of the water in the thin tube stops falling and the floor remains undone.
The pressure exerted by the piston on the solution is equal to the osmotic pressure, which is read by the pressure gauge. In this method there is no direct pressure on the membrane, so high osmotic pressure can also be measured.
What Are The Different Types Of Osmosis
The different types of osmosis include:
Also Check: How To Find Half Life In Chemistry
Osmosis And Osmotic Pressure
Note: this document will print in an appropriately modified format
On this page:
Osmotic pressure is the fourth member of the quartet of colligative properties that arise from the dilution of a solvent by non-volatile solutes. Because of its great importance, we are devoting a separate section to this topic with special emphasis on some of its many practical applications.
Water Transport In Plants: Osmosis Pushes Hydrogen
Osmotic flow plays an important role in the transport of water from its source in the soil to its release by transpiration from the leaves, it is helped along by hydrogen-bonding forces between the water molecules. Capillary rise is not believed to be a significant factor.
Water enters the roots via osmosis, driven by the low water concentration inside the roots that is maintained by both the active transport of ionic nutrients from the soil and by the supply of sugars that are photosynthesized in the leaves. This generates a certain amount of root pressure which sends the water molecules on their way up through the vascular channels of the stem or trunk. But the maximum root pressures that have been measured can push water up only about 20 meters, whereas the tallest trees exceed 100 meters. Root pressure can be the sole driver of water transport in short plants, or even in tall ones such as trees that are not in leaf. Anyone who has seen apparently tender and fragile plants pushing their way up through asphalt pavement cannot help but be impressed!
But when taller plants are actively transpiring (losing water to the atmosphere], osmosis gets a boost from what plant physiologists call cohesion tension or transpirational pull. As each H2O molecule emerges from the opening in the leaf it pulls along the chain of molecules beneath it. So hydrogen-bonding is no less important than osmosis in the overall water transport process.
Don’t Miss: Holt Mcdougal Geometry Worksheet Answer Key
The Formula For Osmotic Pressure
We can determine the Osmotic pressure with the help of the following formula:
Where,
| The temperature on the Kelvin scale. |
Dutch chemist Jacobus vant Hoff gave this relationship between the osmotic pressure of a solution and the molar concentration of its solute. However, this equation holds for the solutions that behave like ideal solutions.
Osmosis And Evolution: Do Fish Drink Water Do They Pee
The following section is a bit long, but for those who are interested in biology it offers a beautiful example of how the constraints imposed by osmosis have guided the evolution of ocean-living creatures into fresh-water species . It concerns ammonia NH3, a product of protein metabolism that is generated within all animals, but is highly toxic and must be eliminated.
But invertebrates that live in fresh water have a problem: the salt concentrations within their bodies are around 1%, much greater than in fresh water. For this reason they have evolved surrounding membranes that are largely impermeable to salts and to water But these organisms must also be able to exchange oxygen and carbon dioxide with their environment. The special respiratory organs that mediate this process, as a consequence of being permeable to these two gases, will also allow water molecules to pass through. In order to protect fresh-water invertebrates from the disastrous effects of unlimited water inflow through the gill membranes, these animals possess special excretory organs that expel excess water back into the environment. Thus in such animals, there is a constant flow of water passing through the body. Ammonia and other substances that need to be excreted are taken up by this stream which constitutes a continual flow of dilute urine.
For the teleosts that live in fresh water, the situation is very much the same as with fresh-water invertebrates they take in and excrete water continuously.
Don’t Miss: Jonathan Thomas Child Of Rage Today
What Happens If You Get The Answer Wrong
Osmotic pressure is critical when dealing with blood cells. If the solution is hypertonic to the cytoplasm of the red blood cells, the cells will shrink through a process called crenation. If the solution is hypotonic with respect to the osmotic pressure of the cytoplasm, water will rush into the cells to try to reach equilibrium. This may cause the red blood cells to burst. In an isotonic solution, red and white blood cells maintain their normal structure and function.
It’s important to remember that there may be other solutes in the solution that affect osmotic pressure. If a solution is isotonic with respect to glucose but contains more or less of an ionic species , these species may migrate into or out of a cell to try to reach equilibrium.
Example: Osmotic Pressure Calculation For A Nonelectrolyte Solution
Osmotic Pressure Problem:
Calculate the osmotic pressure exhibited by a 0.10 mol L-1 sucrose solution at 20oC.
Osmotic Pressure Problem Solution:
= cRTR = 0.0821 L atm K-1mol-1 T = 20oC = 20 + 273 = 293 K
= 0.10 x 0.0821 × 293 = 2.4 atm
= cRTR = 8.314 J K-1mol-1 T = 20oC = 20 + 273 = 293 K
= 0.10 × 8.314 x 293 = 243.6 kPa
Do you know this?
Don’t Miss: Ccl4 Electron Pair Geometry
Chemistry End Of Chapter Exercises
Which is/are part of the macroscopic domain of solutions and which is/are part of the microscopic domain: boiling point elevation, Henrys law, hydrogen bond, ion-dipole attraction, molarity, nonelectrolyte, nonstoichiometric compound, osmosis, solvated ion?
What is the microscopic explanation for the macroscopic behavior illustrated in ?
The strength of the bonds between like molecules is stronger than the strength between unlike molecules. Therefore, some regions will exist in which the water molecules will exclude oil molecules and other regions will exist in which oil molecules will exclude water molecules, forming a heterogeneous region.
Sketch a qualitative graph of the pressure versus time for water vapor above a sample of pure water and a sugar solution, as the liquids evaporate to half their original volume.
A solution of potassium nitrate, an electrolyte, and a solution of glycerin 3), a nonelectrolyte, both boil at 100.3 °C. What other physical properties of the two solutions are identical?
Both form homogeneous solutions their boiling point elevations are the same, as are their lowering of vapor pressures. Osmotic pressure and the lowering of the freezing point are also the same for both solutions.
What are the mole fractions of H3PO4 and water in a solution of 14.5 g of H3PO4 in 125 g of water?
Outline the steps necessary to answer the question.
Answer the question.
What are the mole fractions of HNO3 and water in a concentrated solution of nitric acid ?
Osmosis And Osmotic Pressure Of Solutions
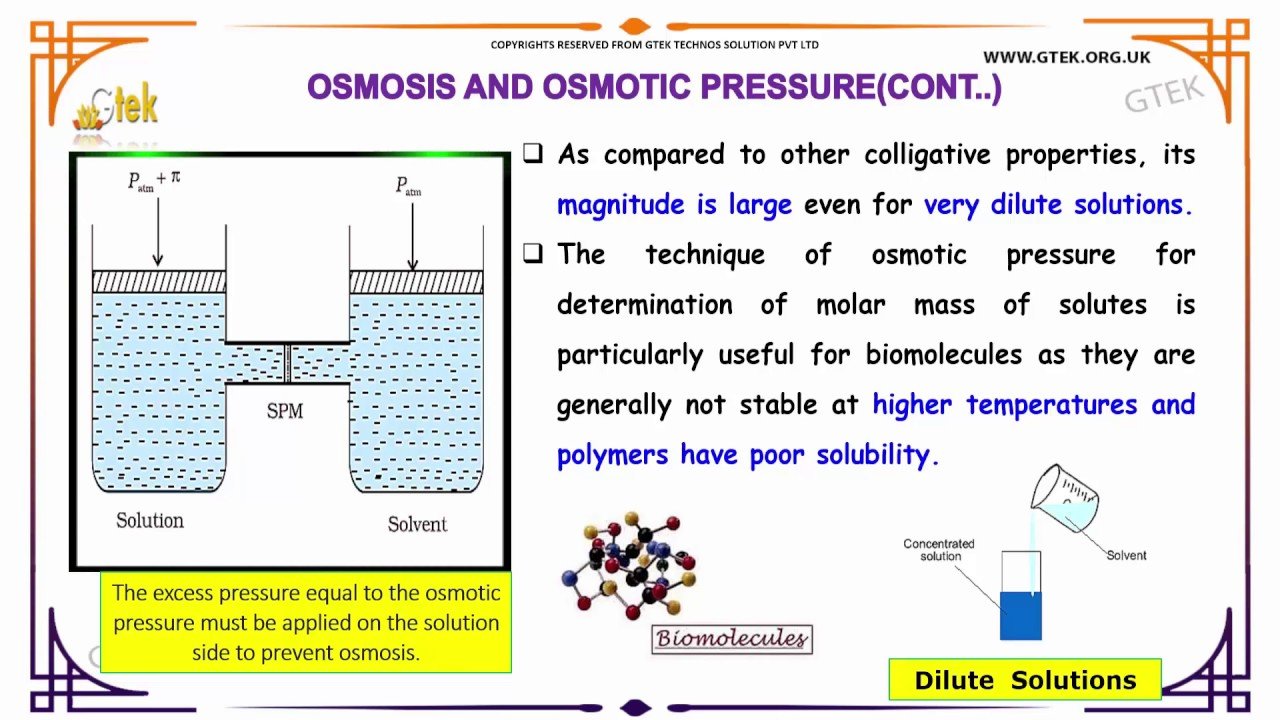
A number of natural and synthetic materials exhibit selective permeation, meaning that only molecules or ions of a certain size, shape, polarity, charge, and so forth, are capable of passing through the material. Biological cell membranes provide elegant examples of selective permeation in nature, while dialysis tubing used to remove metabolic wastes from blood is a more simplistic technological example. Regardless of how they may be fabricated, these materials are generally referred to as semipermeable membranes.
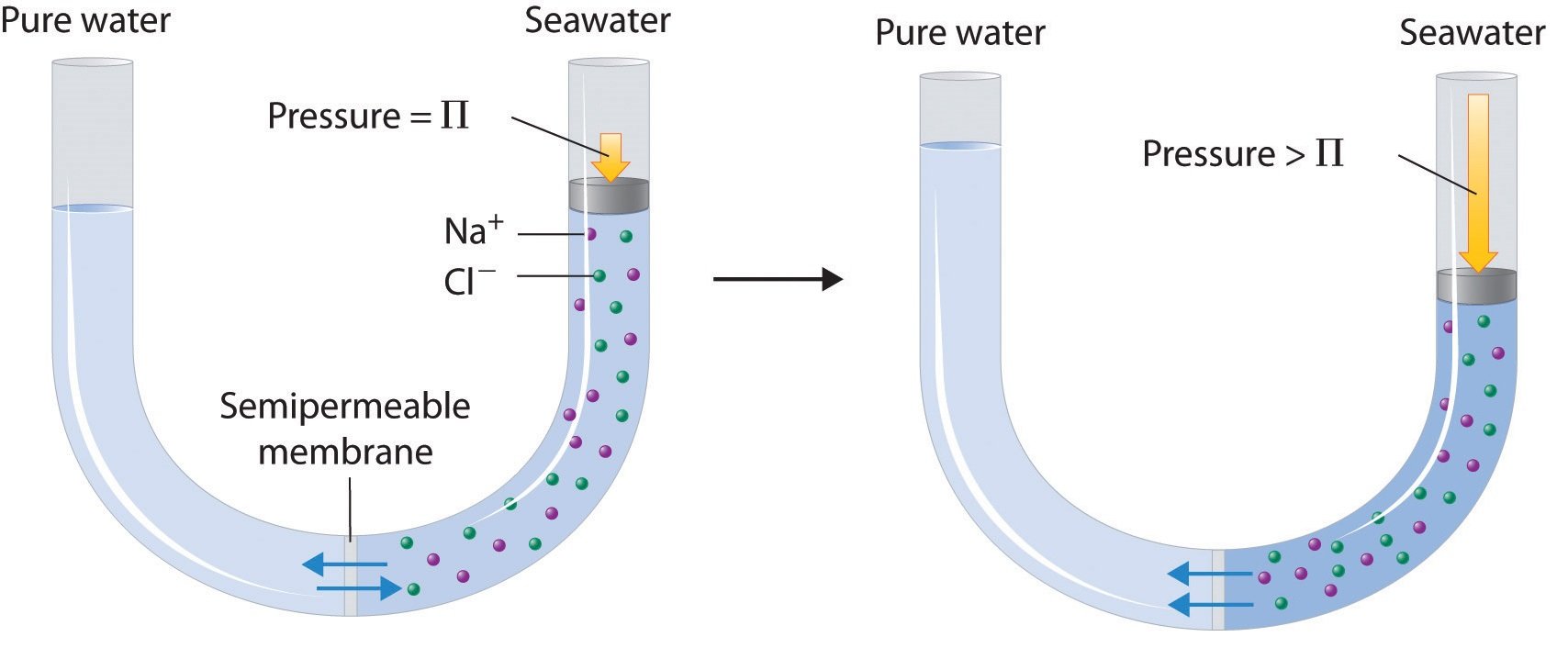
Consider the apparatus illustrated in , in which samples of pure solvent and a solution are separated by a membrane that only solvent molecules may permeate. Solvent molecules will diffuse across the membrane in both directions. Since the concentration of solvent is greater in the pure solvent than the solution, these molecules will diffuse from the solvent side of the membrane to the solution side at a faster rate than they will in the reverse direction. The result is a net transfer of solvent molecules from the pure solvent to the solution. Diffusion-driven transfer of solvent molecules through a semipermeable membrane is a process known as osmosis.
where R is the universal gas constant.
Calculation of Osmotic Pressure Assuming ideal solution behavior, what is the osmotic pressure of a 0.30 M solution of glucose in water that is used for intravenous infusion at body temperature, 37 °C?
5.3 atm
You May Like: Michael Jacksons Biological Son
Difference Between Diffusion And Osmosis
If a layer is made of copper sulphate solution in a beker and the second layer of water by carefully pouring water over it, initially these two layers look different, but after some time these layers look different. And the entire fluid of the beker forms a homogeneous mixture.
This is caused by diffusion. In the process of diffusion, the particles of solute move from the solution of the more concentrated solution to the solution of less concentration and the particles of solvent go from the solution of less concentration to the solution of more concentration.
The main differences between diffusion and osmosis are the following.
Biology Notes
· The presence of a semi permeable membrane is necessary in the action of osmosis while diffusion occurs without any membrane.
· In the action of osmosis the flow of molecules is only in one direction while in the action of diffusion the flow of molecules is on both sides.
· In the process of osmosis, only solvent molecules move from a low concentration to a more concentrated solution, whereas in the process of diffusion, both solute and solvent molecules move in opposite directions.
Understanding Osmotic Pressure What Is Osmosis
The term osmosis refers to the movement of solvent molecules through a semipermeable membrane from a region where the solute concentration is low to a region where the solute concentration is high. Eventually, an equilibrium is established between the two sides of the semipermeable membrane .
Important note: The semipermeable membrane only allows the movement of solvent molecules through it solute particles cannot pass through it.
If sufficient pressure is applied to the solution side of the semipermeable membrane, the process of osmosis is halted. The minimum amount of pressure required to nullify the process of osmosis is called osmotic pressure.
In the illustration provided above, it can be observed that the solvent molecules tend to pass through the semipermeable membrane into the solution side until the osmotic pressure is applied to the solution side.
You May Like: What Does Standardization Mean In Chemistry
How To Calculate Osmotic Pressure
If you want to find osmotic pressure, simply follow the steps below.
Choose a solute to analyze for example, sodium sulfate .
Copy the values of dissociation factor n, molecular weight M and osmotic coefficient from the list below. In this case, n = 3, M = 142 and = 0.74.
Decide on the temperature of the environment in which osmosis takes place for instance 30 °C, what is equivalent to 303.15 K.
If you know the molar concentration of your solution, you can input it directly into the osmotic pressure calculator. If not, you need to determine the mass of the solute m and the total volume of the solution V , as shown in step 5.
Calculate the molar concentration of your solution:
c = m / = 1 / = 0.07 mol/L.
Substitute all of this data into the osmotic pressure equation or simply input it into our osmotic pressure calculator to obtain a result in this case, the pressure is equal to 3940.56 hPa.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Read Also: Beth Thomas Age
What Is Pressure In Chemistry And Gases
PRESSURE is a force exerted by the substance per unit area on another substance. The pressure of a gas is the force that the gas exerts on the walls of its container. When you blow air into a balloon, the balloon expands because the pressure of air molecules is greater on the inside of the balloon than the outside.