Reactions Of Manganese In The World Around Us
Manganese is very chemically active and it has the ability to react with various elements in chemistry which we see on a day to day basis that allow for its diversity in function and uses. Because of its valence electron configuration, it allows us to use it in different and unique ways that typically other elements cannot be used in. In biological systems manganese is a crucial component of vitamin \.
The pure metal is produced from its most common compound —10th most abundant compound in the earth’s crust). It can be reduced chemically or refined electrolytically. The element has at least 5 stable oxidation states with distinctive colors . It is commonly encountered in the laboratory as the compound \ which is a strong oxidizing agent. \ catalyzes the decomposition of \ and is sometimes used for the small scale production of oxygen gas in the lab.
What Does Mn Stand For Chemistry
We compiled queries of the MN abbreviation in Chemistry in search engines. The most frequently asked MN acronym questions for Chemistry were selected and included on the site.
We thought you asked a similar MN question to the search engine to find the meaning of the MN full form in Chemistry, and we are sure that the following Chemistry MN query list will catch your attention.
Chemical Heterogeneity Of Mg Mn Na S And Sr In Benthic Foraminiferal Calcite
- 1LPG UMR CNRS 6112, University of Angers, UFR Sciences, Angers, France
- 2European Synchrotron Radiation Facility, Grenoble, France
- 3Sorbonne Universités, UPMC Univ Paris 06, CNRS, UMR 8220, Laboratoire d’Archéologie Moléculaire et Structurale , Paris, France
- 4Department of Marine Geosciences, Charney School of Marine Sciences, University of Haifa, Haifa, Israel
- 5The Interuniversity Institute for Marine Sciences, Eilat, Israel
- 6Department of Ocean Systems, NIOZ-Royal Netherlands Institute for Sea Research and Utrecht University, Den Burg, Netherlands
- 7Faculty of Geosciences, Utrecht University, Utrecht, Netherlands
- 8Faculty of Agricultural Sciences, Autonomous University of Chihuahua, Chihuahua, Mexico
- 9Department of Geology, Lund University, Lund, Sweden
Read Also: Fsa Algebra 1 Eoc Review Functions And Modeling Answer Key
Green Chemistry And Engineering Innovators
MPCA recognizes the success of two Green Chemistry & Engineering Internship grant recipients at the 2018 MN Cup, a free, annual competition that seeks to support and accelerate development of the best breakthrough ideas from across the state.
Competing in the Energy/CleanTech/Water division, these companies and their innovative products rose to the top among the Minnesota Cups impressive field of over 600 entries.
- The CD3 boat-cleaning station developed by Connect Ecology to protect Minnesota waters from invasive species won the division.
- remooble was the first runner-up for their development of non-toxic and effective chemical removers of inks, paints and adhesives.
CD3s Mark Apfelbacher expressed appreciation of MPCA’s support. In our early stages, the MPCA internship grant helped us pick safer, low-impact materials and coatings for the CD3 boat-cleaning station. Product innovators should really consider applying.
Started in 2005, the MN Cup is the largest statewide startup competition in the country.
We are pleased that the Green Chemistry & Engineering grants can play a small part in growing Minnesotas innovation and entrepreneurial ecosystem.
Tuesday, October 9, 2018
Variability Of Mn Speciation And Distribution In Foraminiferal Test Walls
![Chemical formula of Mn[C10H6(OH)(COO)]2×2H2O.](https://www.tutordale.com/wp-content/uploads/chemical-formula-of-mnc10h6ohcoo22h2o-download.png)
Coordination of Mn in Ammonia sp. and B. marginata
Finally, measurements by XRF were not able to detect changes between low and high Mn bands inside the calcite, but we did observe in some cases a coating on the inside of the foraminiferal test and within the pores . This coating is enriched in Mn and shows a patchy distribution. XANES spectra from these areas are very different from the spectra acquired in the calcite layers and present the same features as the organic references analyzed. To our opinion, this coating would correspond to the remaining cytoplasm enriched in Mn , even though specimens were cleaned with NaOCl. This highlights the importance of the organic removal step, because especially in the case of bulk analyses, the remaining cytoplasm of such an enriched Mn coating would result in artificially higher foraminiferal Mn/Ca values.
Microdistribution of Mn in Foraminiferal Calcite
Don’t Miss: Geometry Segment Addition Postulate Worksheet
How Molecular Weight Is Determined
Empirical data on the molecular weight of a compound depends on the size of the molecule in question. Mass spectrometry is commonly used to find the molecular mass of small to medium-sized molecules. The weight of larger molecules and macromolecules is found using light scattering and viscosity. Specifically, the Zimm method of light scattering and the hydrodynamic methods dynamic light scattering , size-exclusion chromatography , diffusion-ordered nuclear magnetic resonance spectroscopy , and viscometry may be used.
Health Effects Of Manganese
Manganese is a very common compound that can be found everywhere on earth. Manganese is one out of three toxic essential trace elements, which means that it is not only necessary for humans to survive, but it is also toxic when too high concentrations are present in a human body. When people do not live up to the recommended daily allowances their health will decrease. But when the uptake is too high health problems will also occur. The uptake of manganese by humans mainly takes place through food, such as spinach, tea and herbs. The foodstuffs that contain the highest concentrations are grains and rice, soya beans, eggs, nuts, olive oil, green beans and oysters. After absorption in the human body manganese will be transported through the blood to the liver, the kidneys, the pancreas and the endocrine glands. Manganese effects occur mainly in the respiratory tract and in the brains. Symptoms of manganese poisoning are hallucinations, forgetfulness and nerve damage. Manganese can also cause Parkinson, lung embolism and bronchitis. When men are exposed to manganese for a longer period of time they may become impotent. A syndrome that is caused by manganese has symptoms such as schizophrenia, dullness, weak muscles, headaches and insomnia. Because manganese is an essential element for human health shortages of manganese can also cause health effects. These are the following effects: – Fatness
Read Also: What Is Figure Ground Perception Psychology
Molecular Weight Versus Molecular Mass
Molecular weight is often used interchangeably with molecular mass in chemistry, although technically there is a difference between the two.;Molecular mass is a measure of mass and molecular weight is a measure of force acting on the molecular mass. A more correct term for both molecular weight and molecular mass, as they are used in chemistry, would be “relative molecular mass”.
Distribution Of Mn Mg S And Na By Electron Probe Microanalysis
Examples of element to calcium maps per species are presented in Figure 5. All three species show characteristic banding patterns and peak intensities, depending on the element of interest. The most pronounced banding can be found in Mg/Ca and Mn/Ca maps, depending in the latter case of the seawater of the culture experiment; that is, Mn banding is most visible for Aq100 and Aq600 and not in Aq10, owing to the limit of detection. Ammonia sp. shows thin bands of 12 m of high E/Ca for Mg, S, Na, and Mn. Distributions of Mg, S, and Na follow the same pattern of thin bands in specimens of Bulimina marginata and Amphistegina lessonii , but Mn/Ca distribution differs . For B. marginata, Mn/Ca is present as a thin highly concentrated band located close to the Mg band but is often heterogeneously distributed within the lamella. In contrast, for A. lessonii, Mn/Ca distribution is characterized by broad bands with either high or low Mn/Ca, corresponding to different lamella of the test wall.
Figure 5. EPMA element distribution maps for three species of benthic foraminifera, Ammonia sp. T6 , Bulimina marginata , and Amphistegina lessonii , from treatment Aq600 . The top maps show information on chamber number , and the asterisk indicates the outer side of the test. Maps are semiquantitative . EPMA, electron probe microanalysis.
Table 2. Average E/Ca per species per treatment of total distribution maps EPMA.
Mg, Na, and S Distribution: Electron Probe Microanalysis Peak Analysis
Also Check: Ccl4 Valence Electrons
Biological Role In Plants
Manganese is also important in photosynthetic oxygen evolution in chloroplasts in plants. The oxygen-evolving complex is a part of photosystem II contained in the thylakoid membranes of chloroplasts; it is responsible for the terminal during the light reactions of , and has a metalloenzyme core containing four atoms of manganese. To fulfill this requirement, most broad-spectrum plant fertilizers contain manganese.
| 0 |
Manganese compounds are less toxic than those of other widespread metals, such as nickel and copper. However, exposure to manganese dusts and fumes should not exceed the ceiling value of 5;mg/m3 even for short periods because of its toxicity level. Manganese poisoning has been linked to impaired motor skills and cognitive disorders.
Permanganate exhibits a higher toxicity than manganese compounds. The fatal dose is about 10;g, and several fatal intoxications have occurred. The strong oxidative effect leads to necrosis of the mucous membrane. For example, the esophagus is affected if the permanganate is swallowed. Only a limited amount is absorbed by the intestines, but this small amount shows severe effects on the kidneys and on the liver.
What Is Manganese Used For In Everyday Life
Manganese is used to make clear glass, to desulfurize and deoxidize steel in steel production and to reduce the octane rating in gasoline. It also is used as a black-brown pigment in paint and as filler in dry cell batteries. Its alloys help stiffen the aluminum in soft-drink cans, according to Chemicool.
Read Also: What Kind Of Math Is On The Ged Test
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Other Oxidation States Of Manganese
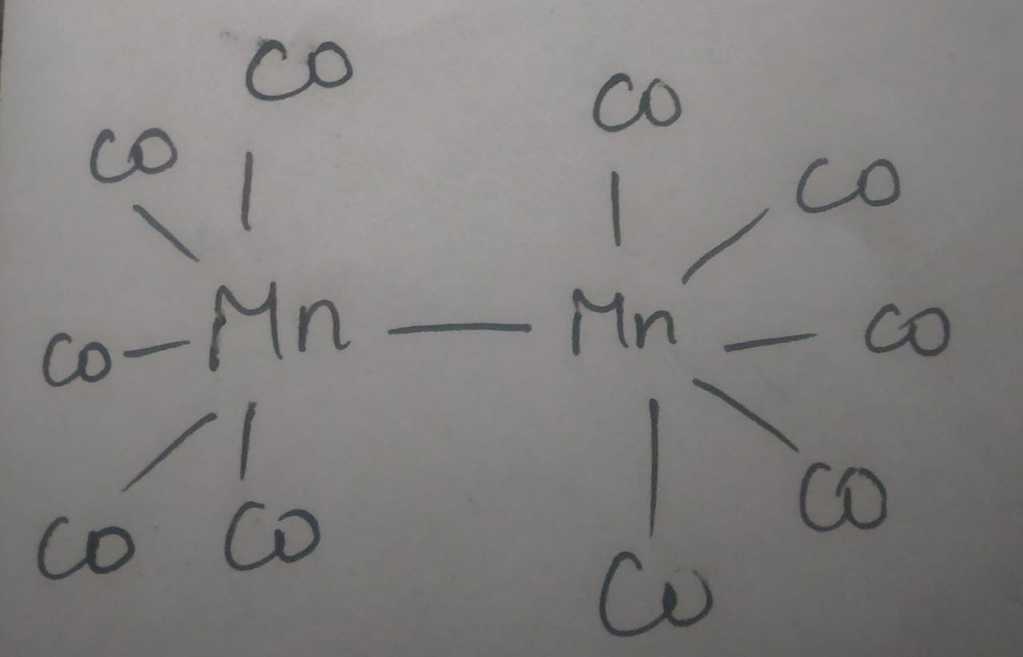
The oxidation state 5+ can be obtained if manganese dioxide is dissolved in molten sodium nitrite. Manganate salts can also be produced by dissolving Mn compounds, such as manganese dioxide, in molten alkali while exposed to air. Permanganate compounds are purple and can give glass a violet color. Potassium permanganate, sodium permanganate, and barium permanganate are all potent oxidizers. Potassium permanganate finds use as a topical medicine . Solutions of potassium permanganate were among the first stains and fixatives to be used in the preparation of biological cells and tissues for electron microscopy.
Arginase
Also Check: What Is Mean Median Mode And Range In Math
What Is The Meaning Of Mn Abbreviation In Chemistry
What is MN definition ?
MN definition is “Meson Nucleon”.
What does MN mean in Chemistry?
MN mean that “Miscellaneous Notes” for Chemistry.
What is MN acronym ?
What is shorthand of Meson Nucleon ?
The shorthand of “Meson Nucleon” is MN.
What is the definition of MN acronym in Chemistry?
Definitions of MN shorthand is “Meson Nucleon”.
What is the full form of MN abbreviation?
Full form of MN abbreviation is “Manganese”.
What is the full meaning of MN in Chemistry?
Full meaning of MN is “Manganese”.
What is the explanation for MN in Chemistry?
Explanation for MN is “Meson Nucleon”.
What is the meaning of MN Abbreviation in Astrology ?
The site does not only include the meanings of the MN abbreviation in Chemistry. Yes, we know your main purpose is explanation of MN abbreviation in Chemistry. However, we thought that besides the meaning of the MN definitions in Chemistry, you can consider astrological information of MN acronym in Astrology. Therefore, the astrological explanation of each word in each MN abbreviation is also included.
MN Abbreviation in Astrology
Sample Molecular Weight Calculation
The calculation for molecular weight is based on the molecular formula of a compound . The number of each type of atom is multiplied by its atomic weight and then added to the weights of the other atoms.
For example, the molecular formula of hexane is C6H14. The subscripts indicate the number of each type of atom, so there are 6 carbon atoms and 14 hydrogen atoms in each hexane molecule. The atomic weight of carbon and hydrogen may be found on a periodic table.
- Atomic weight of carbon: 12.01
- Atomic weight of hydrogen: 1.01
molecular weight = + so we calculate as follows:
- molecular weight = +
- molecular weight of hexane = 72.06 + 14.14
- molecular weight of hexane = 86.20 amu
Read Also: Ccl4 Lewis Structure Molecular Geometry
Manganese Speciation By Micro
The first observations of the combined spectra from the different locations, that is, internal coating and calcite locations with reference spectra, suggests that Mn is systematically under a Mn speciation. The results of the PCA performed on normalized spectra of all samples plus the set of references are presented in Figure 10A. The first two axes of the PCA explain 77% of the variability of the data. The analysis highlights the clear difference in the chemical environment of Mn between the internal coating location and the measurements performed in the calcite itself . The averaged spectra for the different locations show that the near-edge features are mainly characterized by two distinct peaks in the calcite, whereas only one large white line peak is observed for the internal coating zone. Regarding the calcite samples, the spectra are in general following the same trend, but the intensities of the near-edge peaks seem to be variable , and the PCA suggests some small differences between the two species and between the two conditions, for example, high Mn calcitePRE locations of Bulimina and Ammonia in Figure 10A and Supplementary Figure 5A.
Oxidation State Of Mn In Mno
I have tried to understand what the oxidation state for Mn in $\ce$ is. So far i have understood that O have the oxidation state of -2 so because there is 4 O the number turns to -8. What happens to the minus at O? and what is the oxidation state for Mn?
The sum of the oxidation states of all of the atoms needs to equal the total charge on the ion.
For example, for $\ce$, if each hydrogen atom has an oxidation state of +1, and the overall charge is +1, then we can solve for the oxidation state of nitrogen:
$$\begin 4 + OS_\ce &= +1\\OS_\ce &= +1 -4 \\OS_\ce &= -3\end$$
Similarly, we can do $\ce$. If each oxygen atom has an oxidation state of -2, and the overall charge is -2, we can solve for the oxidation state of sulfur:
$$\begin 4 + OS_\ce &= -2\\OS_\ce &= -2 -4 \\OS_\ce &= +6\end$$
Also Check: Geometry Segment Addition Postulate Worksheet
Does The Human Body Use Manganese
Manganese is a trace mineral. It is vital for the human body, but people only need it in small amounts. Manganese contributes to many bodily functions, including the metabolism of amino acids, cholesterol, glucose, and carbohydrates. It also plays a role in bone formation, blood clotting, and reducing inflammation.
Professional Learning And Coming Events
Recommended Reading: Ccl4 Shape
Resources For Instruction And Learning
Support for 2009 Science StandardsThe Frameworks for Minnesota Mathematics and Science StandardsView the Frameworks for Minnesota Mathematics and Science Standards.Support for the 2019 Science Standards:A Framework for K-12 Science EducationNext Generation Science StandardsScience Lab SafetyView the checklist at the Minnesota Department of Public SafetyScience Best Practices/Science SafetyFrameworksScience Teachers Association Safety ResourcesGeographic Information SystemsFor more information go to the MDE GIS webpageMinnesota Science Teachers AssociationMnSTANational Science Teaching AssociationNSTA
- Answers questions about the final draft of the science standards revision document.
-
An Equal Opportunity Employer and Service Provider;
Elemental Microdistribution In Foraminiferal Tests
Electron Probe Microanalysis
Specimens of A. lessonii from the experiment described in this study, together with specimens of Ammonia sp. and B. marginata from the study of Barras et al. , were measured on a field emission EPMA at Utrecht University to investigate the intra-test incorporation of Mg, S, Na, and Mn. Specimens of each species from all of the four culture conditions were embedded under vacuum in resin using 2.5-cm epoxy plugs. Samples were polished using increasingly finer sanding paper, resulting in exposure of cross section of chamber walls. In the final polishing step, a diamond emulsion with grains of 0.04 m was used to create a smooth surface. Scanning electron microscope images were taken with a tabletop Hitachi TM3000 at the Royal Netherlands Institute for Sea Research to find areas of interest .
Figure 1. Examples of SEM images of polished specimens of Ammonia sp. T6 , Bulimina marginata, and Amphistegina lessonii. Chambers precipitated during the experiment are recognized owing to pre-staining and are numbered from final to older chambers . Scale bar is 100 m. SEM, scanning electron microscope.
Nanoscale Secondary Ion Mass Spectrometry Analyses
Micro-X-Ray Fluorescence and Micro-X-Ray Absorption Near-Edge Structure Analyses
Read Also: Eoc Fsa Practice Test Answers