Hydration/dehydration Reactions In The Solid Earth
In Earth science, the term water commonly includes a range of H-bearing compounds such as molecular H2O, hydroxyl groups , or simply H. This water can be incorporated in rocks in multiple ways, such as in hydrous minerals, in nominally anhydrous minerals, in fluid inclusions, or adsorbed onto mineral surfaces without entering the structure of the mineral. Hydrous minerals can host water as either molecular H2O or OH, or in both forms, and include a large variety of mineral groups such as clays, amphiboles, micas, chlorite, serpentines, lawsonite, and many others, some capable of hosting more than 10 wt% water. Among the most important hydrous minerals is serpentine , which forms through the hydration of olivine, ranging in composition from Mg2SiO4 to Fe2SiO4, as described by the model reaction:
The serpentinization reaction may also involve oxidation of Fe2+ in iron-containing olivine and other minerals such as pyroxenes, and its partitioning among minerals such as magnetite, brucite, and serpentine, for example :
Nominally anhydrous minerals are minerals that do not contain water in their formula by definition but where H or, more rarely molecular H2O, can be incorporated in structural defects such as cation vacancies and charge deficiencies . Typical examples are olivine, pyroxenes, and garnet, all of which can host several hundred parts per million of water.
What Is Hydration In Chemistry
In chemistry, hydration reaction is fundamentally a chemical reaction where a substance combines with water.
However, in organic chemistry, the hydration reaction is one in which water is added to an unsaturated substrate .
This type of reaction is how industrial processes make isopropanol, ethanol, and 2-butanol.
Shakespeare Sonnet 3: How Can My Muse Want Subject To Invent
Each copper sulphate unit can attach to five water molecules, so its sometimes called copper sulphate pentahydrate when its hydrated. The formula of the hydrated form is CuSO4. 5H2O. The dot after the formula for copper sulphate indicates bonds with water molecules. Research suggests that the nature of these bonds is not as simple as was once thought.
Don’t Miss: What Is Biological Treatment For Rheumatoid Arthritis
Application Of Hydration Enthalpy
One application of enthalpy of hydration is the reaction of cement with water. The reaction being exothermic releases a large amount of heat. This heat released becomes significant in mass constructions like building dams and big structures. For the construction of massive concrete blocks, large quantities of cement are used.
During the process of setting the heat is released. The outer surfaces of the block cool relatively faster than the interior, this creates a thermal gradient in the block and can initiate cracks that lead to failure of the structure. To avoid this problem low heat types of cement are preferred for massive construction, cement with pozzolanic admixtures preferably fly ash or slag and also using ice instead of water to prepare concrete.
Features And Significance Of Hydration In Chemistry
The nature of the process and its main features
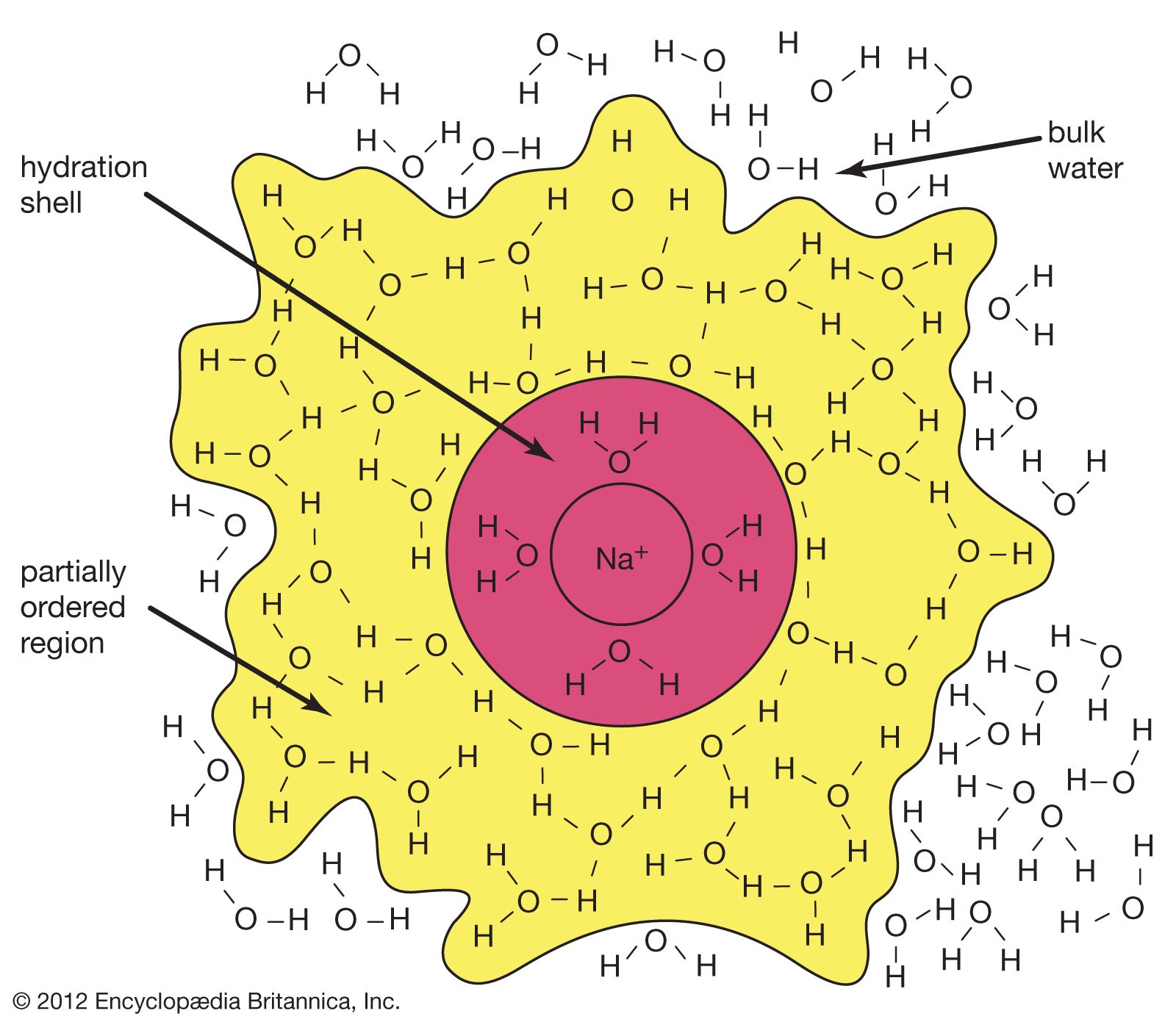
Hydration in chemistry has significance for many processes. Hydration in chemistry is the attachment of water molecules to the molecule of another substance, if were talking in general terms. It is one of the main topics of study, as other topics in chemistry are based on this knowledge.
The main reason for this is that many reactions and processes take place with water. In order to get a detailed understanding of hydration, we should not only understand the nature of the process, but also its significance.
You May Like: Does Mj Have Any Biological Kids
Relating Hydration Energy To Lattice Enthalpy
In the discussion of lattice energy, we consider the ions separated into a gas form whereas in the dissolution process, the ions are also separated, but this time into ions dispersed in a medium with solvent molecules between ions. The medium or solvent has a dielectric constant. The molar enthalpy of solution, \, is the energy released when one mole solid is dissolved in a solvent. This quantity, the enthalpy of crystallization, and energy of hydration forms a cycle. Taking the salt \ as an example, the following relationship is obvious,
from the following diagram.
The term enthalpy of crystallization is used in this diagram instead of lattice energy so that all the arrows point downward. Note that enthalpy of crystallization \, and energy of crystallization, \ refer to the same quantity, and they are used interchangably. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve the dissolving is an endothermic reaction. The energy levels of solids and solutions reverse in order of height.
Gas Hydrates And Their Potential Uses
Chunks of gas hydrates look like lumps of ice and appear to be crystalline solids. The building blocks of the hydrates are made at low temperature and high pressure when water molecules surround a gas molecule, forming a frozen mesh or cage. The gas is often methane, in which case the name methane hydrate may be used for the hydrate, but it may also be carbon dioxide or another gas. The methane is produced by bacterial decay of dead plants and animals. Methane has the formula CH4.
Gas hydrates have been located around the world. They form in sediments at the bottom of deep oceans and lakes and are also found on land in permafrost. Methane hydrates have the potential to be an excellent source of energy. In fact, some researchers estimate that the total amount of energy trapped in the world’s gas hydrates may be greater than the total energy present in all known fossil fuels on Earth. If a gas hydrate is lit by a match or another flame, it will burn like a candle.
Don’t Miss: Is Living Environment The Same As Biology
Epsom Salts Borax And Glauber’s Salt
Epsom salts are named after the town of Epsom in England, where they were discovered. They are used in different formulations as bath salts and as soil fertilizer. Many people claim that magnesium from the chemical can be absorbed through the skin. There is little scientific evidence for this process, however.
Borax is used as a household cleaner and is present in low concentrations in some cosmetics. There are multiple concerns about its safety. It mustnt be ingested and should be kept out of reach of children and pets.
Glaubers salt is named after Johann Rudolf Glauber, a German-Dutch chemist and apothecary who lived in the seventeenth century. Glauber discovered sodium sulphate and also discovered that it acts as a laxative in humans.
Societal Impact Of Hydration/dehydration Reactions
Hydration and dehydration reactions feature prominently in modern chemistry and are essential steps in the construction of our cities and production of numerous compounds, including various aldehydes, alcohols, and precursors of polymers. These reactions have been extensively leveraged in modern chemistry both for their applications in the synthesis of organic molecules for pharmaceutical applications and in the industrial productions of modern materials. For example, the hydration of Portland cement to form concrete is a centerpiece of the modern construction industry. This hydration is highly exothermic, similar to other hydration reactions, and part of the chemistry of which is summarized in the following reaction :
Tricalcium silicate is the main constituent of Portland cement accounting for 5070% of the final mass, and it is one of the most reactive silicates in water . Its hydration is responsible for the setting and initial strengthening of cement paste. Similar silicate hydration reactions, albeit very different in their molecular dynamics, are prevalent in natural and technological processes.
Recommended Reading: What Is The Physical Geography Of Africa
Pozzolanic Reaction Of Rha
During the hydration process of cement, two primary components of cement, namely tricalcium silicate and dicalcium silicate , react with water to form hydration products, namely CSH and CH . At the same time, hydration of tricalcium aluminate and tetra calcium aluminoferrite also proceeds to generate the corresponding calcium aluminate hydrate and calcium aluminate ferrite hydrate, respectively.
The gel phase of cement products refers to the poorly crystallized CSH and CAH, which are responsible to form a continuous binding matrix and hardened into cement stone with age. The two components are the key contributors to the strength development in the cement body.
As for the crystalline hydration products CH and ettringite, they are randomly distributed in the hydration products and act as the frame of the cement gel matrix. However, excessive CH content is detrimental to cement strength development, since CH prefers to grow along with one crystalline orientation. It is widely recognized that incorporating pozzolanic material into a cement mix accelerates the consumption of the released CH during the cement hydration, due to the pozzolanic reaction . In the pozzolanic reaction, CH reacts with the SiO2 or aluminosilicates framework to form CSH, CAH, and CAFH.
Dehydration Reaction Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A dehydration reaction is a chemical reaction between two compounds where one of the products is water. For example, two monomers may react where a hydrogen from one monomer binds to a hydroxyl group from the other monomer to form a dimer and a water molecule . The hydroxyl group is a poor leaving group, so Bronsted acid catalysts may be used to help to protonate the hydroxyl to form -OH2+. The reverse reaction, where water combines with hydroxyl groups, is termed hydrolysis or a hydration reaction.
Chemicals commonly used as dehydrating agents include concentrated phosphoric acid, concentrated sulfuric acid, hot ceramic and hot aluminum oxide.
A dehydration reaction is the same as a dehydration synthesis. A dehydration reaction may also be known as a condensation reaction, but more properly, a dehydration reaction is a specific type of condensation reaction.
Also Check: Chapter 6 Mid Chapter Test Glencoe Algebra 2
Interesting And Important Chemicals
Hydrates are interesting chemicals that are often very useful. Exploring their nature, properties, and behaviour is important. Gas hydrates are particularly interesting and are attracting the attention of many researchers. They could become very helpful in our future. There is much to learn about the best ways to use them and about safety procedures, however. Hopefully, their effects on our lives will be beneficial instead of harmful.
What Is The Enthalpy Of Hydration
Enthalpy of hydration, \, of an ion is the amount of heat released when a mole of the ion dissolves in a large amount of water forming an infinite dilute solution in the process,
where Mz+ represents ions surrounded by water molecules and dispersed in the solution. The approximate hydration energies of some typical ions are listed here. Figure \ illustrates the point that as the atomic numbers increases, so do the ionic size, leading to a decrease in absolute values of enthalpy of hydration.
| Ion | |
|---|---|
| Hg2+ | -1824 |
From the above table, an estimate can be made for the hydration energy of sodium chloride. The hydration energy of an ionic compound consists of two inseparable parts. The first part is the energy released when the solvent forms a coordination compound with the ions. This energy released is called the Enthalpy of ligation, \. The processes related to these energies are shown below:
The second step is to disperse the ions or hydrated ions into the solvent medium, which has a dielectric constant different from vacuum. This amount of energy is called energy of dispersion, \. Therefore,
Also Check: What Does Origin Mean In Math Terms
General Features For Hydration Mechanisms
In addition to the two examples described above, IR spectrometric methods have been applied to a few proteins, such as bovine serum albumin or lysozyme . They provided hydration mechanisms similar to those described above, but the relation between the determined structure of the H-bond network at a definite hygrometry and properties of macromolecules requires a deeper analysis, in view of the complexity of such macromolecules. It means that a greater number of data are necessary to establish the structure of the H-bond network in active proteins and its precise role. Clues on this role have nevertheless been given in studies on cryo- and lyoprotection of proteins described in the following subsection.
J. Murton, in, 2013
Asthmaandallergies Can Be Linked To Dehydration
Dr. Batmanghelidj discovered that when the body is dehydrated, histamine begins to ration water. This in turn increases the histamine and allergic response and reduces immunity.
Chronic dehydration triggers the release of histamine in people with asthma, which leads to inflammation and bronchial constriction.
You May Like: What Is Duodenum In Biology
Dehydration: Polymerization Of Biological Molecules
In resting cells, non-water biomass is distributed among proteins , nucleic acids , lipids , polysaccharides , and small metabolites plus ions . Of these, the three major classes of biological macromolecules are universally polymerized by dehydrative condensation from their constituent monomers as depicted in Figure 2a, and while lipids are not polymerized by dehydration, vicinal dehydration is a critical intermediate step in lipid biosynthesis .
Examples of important biochemical dehydration reactions. Reactions are depicted as schema intended to highlight the dehydration reaction and not as a strict representation of the cellular processes, which both involve enzymes and activating chemistries. Anabolic dehydration reactions are responsible for the formation of each of the three major classes of biopolymers . The converse hydration reactions are used for the breakdown of these polymers in catabolic metabolism. Phosphorylation and polyphosphorylation are dehydration reactions critical for activating other biochemical reactions, as well as cellular regulation. Vicinal dehydration and hydration reactions are crucial in core metabolism.
Examples Of Dehydration Synthesis Reaction
1. Biochemical reactions are one of the best examples of dehydration synthesis reactions. Amino acids are building blocks of proteins as they polymerize to form peptides and polypeptides. Amino acids have two functional groups amino -NH2 and carboxylic group . They react to form an amide linkage with the elimination of water molecules.
Hence, peptide formation is an example of a dehydration synthesis reaction.
2. Another example of dehydration synthesis is the formation of polysaccharides. The monomer units for polysaccharides are monosaccharides which are polyhydroxy carbonyl compounds. Monosaccharides are bonded with each other through glycosidic linkage. This linkage is formed by the reaction of OH groups of two monomer units with the elimination of water molecules.
Formation of maltose is an example of a dehydration synthesis reaction. Two alpha-glucose units form a glycosidic linkage with elimination of water molecules to form one maltose molecule.
So we can say that a dehydration synthesis always involves two steps.
Don’t Miss: What Is Groynes In Geography
What Is The Hydrate Chemistry Naming System
There are specific rules to follow for writing formulas of and naming inorganic hydrates. Because the water molecules aren’t part of the compound’s actual structure, this affects the way chemical formulas of inorganic hydrates are written. An example of a hydrate formula is $CaCl_2$ $2H_2O$. The dot separating the $CaCl_2$ from the two water molecules isn’t a multiplication symbol. It shows that the water molecules aren’t bonded to the compound, and it’s therefore a hydrate.
In order to properly name a hydrate, first you give the name of the salt. The second part of the name begins with a prefix. The prefix is determined by the number of water molecules in the hydrate which is itself determined by the salt. You then add the word “hydrate” to the prefix to give the complete hydrate name. For the formula given above, it’s hydrate name is calcium chloride dihydrate. The prefix “di” is used because it has two water molecules.
Below are the prefixes for different numbers of water molecules.
| Number of H20 molecules |
Here are some examples of hydrate formulas along with their names so you can get a better sense of hydrate compound names.
- $CuSO_4$ $5H_2O$ : Copper sulfate pentahydrate
- $CoCl_2$$6H_2O$: Cobalt chloride hexahydrate
- $BeSO_4$ $4H_2O$: Beryllium sulfate tetrahydrate
- $K_2CO_3$ $1.5H_2O$: Potassium carbonate sesquihydrate
- $CaSO_4$ $0.5H_2O$: Calcium sulfate hemihydrate
What Do You Mean By Hydration Enthalpy Explain With An Example
Since smaller atoms can accommodate the increasing number of water molecules surrounding them and become hydrated, the hydration enthalpy would be larger as the ions get smaller. The size of an atom rises as the hydration enthalpy lowers down the group due to the inclusion of additional valence shells.
In addition, when the size of a cation grows, the hydration enthalpy falls. The lattice enthalpy, on the other hand, drops quicker than the hydration enthalpy due to a square factor. As a result, the solubility of the second group of hydroxides rises as we continue down the group.
Many ionic molecules or compounds are not soluble in non-aqueous solutions however, their solubility is high in water. We all know that water is one of the polar molecules, with a half negative charge from oxygen and a positive charge from hydrogen.
The three factors that influence hydration enthalpy are:
- Enthalpy of Hydration
The quantity of energy produced when a single mole of ions undergoes hydration is hydration energy or hydration enthalpy. Hydration is a kind of solvation. Hydration energy is a form of dissolution of energy where the solvent is water.
Recommended Reading: How To Solve Algebra Problems
Possible Dangers Of Gas Hydrates
Not everyone is excited by the discovery of gas hydrates. Some people think that they could be a natural hazard rather than a natural resource. Researchers are currently trying to find the most effective way to extract methane molecules from their water cages. Some people worry that as a result of the extraction, methane will enter the atmosphere and affect the Earth’s climate. It’s thought that methane in the atmosphere contributes to global warming.
Gas hydrates can block natural gas pipelines and may sometimes be a drilling hazard. Another problem could result from the fact that the hydrates cement ocean sediments together. If the hydrates in a large area melt, the sediments could move. This might produce a landslide that may cause a tsunami.