Fun Facts On Formal Charge
-
In organic chemistry, convention governs that formal charge is essential for depicting a complete and correct Lewis-Kekulé structure. However, the same does not apply to inorganic chemistry.;;
-
The structure variation of a molecule having the least amount of charge is the most superior.;
-
The differences between formal charge and oxidation state led to the now widely followed and much more accurate valence bond theory of Slater and the molecular orbital theory of Mulliken.
1. Define formal charge. Why is it important to calculate it?
Ans: A method of formal charge definition is to state that it is a theoretical charge present on individual atoms of a polyatomic molecule. The formal charge on an atom exists because of unfulfilled orbital configuration. It is theoretical and considered fake as the real, physical charge on a molecule or ion is distributed throughout its structure. However, its calculation is pretty essential as it leads us to make several predictions. The lesser the formal charge on a particular possible structure of a molecule, the more stable it is. Therefore, it is more likely that this structure will dominate a chemical reaction. In this way, we can predict the major product of a reaction. The concept of formal charges also helps us justify many phenomena.
2. How to calculate formal charges?
Observing Charge Distribution In Molecules
Microscopists have mapped the distribution of charge across a single organic molecule for the first time
The distribution of charge across a single molecule has been imaged for the first time by Swiss scientists. It is hoped that this work may eventually lead to electronic devices consisting of organic molecules.;
Fabian Mohn and his colleagues at IBM Research-Zurich in Switzerland are well known for their scanning probe microscopy research. So far, they have used atomic force microscopy to view all the atoms in a molecule and measure the charge on single atoms, and have also used scanning tunnelling microscopy to see molecular orbitals. Now, they have used a third mode of the same microscope-Kelvin scanning probe microscopy – to image the charge distribution in a single organic molecule: naphthalocyanine.;
The positive and negative charged arms of X-shaped naphthalocyanine can be clearly seen, and compare well with theoretic charge distribution images
Naphthalocyanine can switch between tautomers on application of an electric current
The next step for the IBM team is to probe the formation of covalent bonds between two molecules. First, you would look at the two molecules separately, and then you would form the bond. After the bond formation you look at how the charge has rearranged in the molecule, says Mohn.;
Nina Notman;
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie; no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Recommended Reading: What Is The Molecular Geometry Of Ccl4
Give Each Student An Activity Sheet
Have students answer questions about the illustration on the activity sheet. Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms & Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually, depending on your instructions.
Explore
Solved Formal Charge Examples
Calculate the formal charge on the following:
O atoms of O3
Cl atom in HClO4- ion
S atom in HSO4- ion
Ans:;
We are showing how to find formal charge of the species mentioned.
Formal charge on O1: 6 6/2 2 = +1
Formal charge on O2: 6 4/2 4 = 0
Formal charge on O3: 6 2/2 6 = -1
Formal charge on Cl atom of HClO4 ion: 7 8/2 0 = 3
Formal charge on S atom of HSO4- ion: 6 8/2 0 = 2
Recommended Reading: What Is The Molecular Geometry Of Ccl4
Importance Of Formal Charge:
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Is Cathode Positive Or Negative In Vaccume Tube Or Cathode Ray Tube
In a vacuum tube or a cathode ray tube, the cathode is the negative terminal.;This is where the electrons enter the device and continue into the tube.;A positive current leaves the device.
In a diode, the cathode is indicated by the pointed end of an arrow symbol.;It is the negative terminal from which the current flows.;Even if current can flow in both directions through a diode, the name is always based on the direction in which current flows most easily.
Read Also: Geometry Segment Addition Postulate Worksheet
Formal Charge Formula And Shortcut For Organic Chemistry
Formal Charge is that pesky concept covered early in organic chemistry and somehow never seems to go away.
Why do I call it pesky? Because the equation in your textbook is long, confusing, and needlessly annoying.
How can you afford that kind of time when working through a multi-step synthesis?
You Cant! You shouldnt have to.
Yet understanding the nature of Formal Charge is a critical component when it comes to mastering organic chemistry reactions and mechanisms. Formal charge helps you understand reaction patterns by showing you why a specific atom attacks, and why its victim accepts the attack.
Importance Of Formal Charge
Now that we know what is formal charge and we are familiar with the process for calculating formal charge, we will learn about its importance.;
-
The formal charge is a theoretical concept, useful when studying the molecule minutely. It does not indicate any real charge separation in the molecule. This concept and the knowledge of what is formal charge’ is vital.
-
Formal charge is crucial in deciding the lowest energy configuration among several possible Lewis structures for the given molecule. Therefore, calculating formal charge becomes essential.
-
Knowing the lowest energy structure is critical in pointing out the primary product of a reaction. This knowledge is also useful in describing several phenomena.
-
The structure of least energy is usually the one with minimal formal charge and most distributed real charge.;
Besides knowing what is formal charge, we now also know its significance.;
Also Check: Eoc Fsa Practice Test Algebra 1
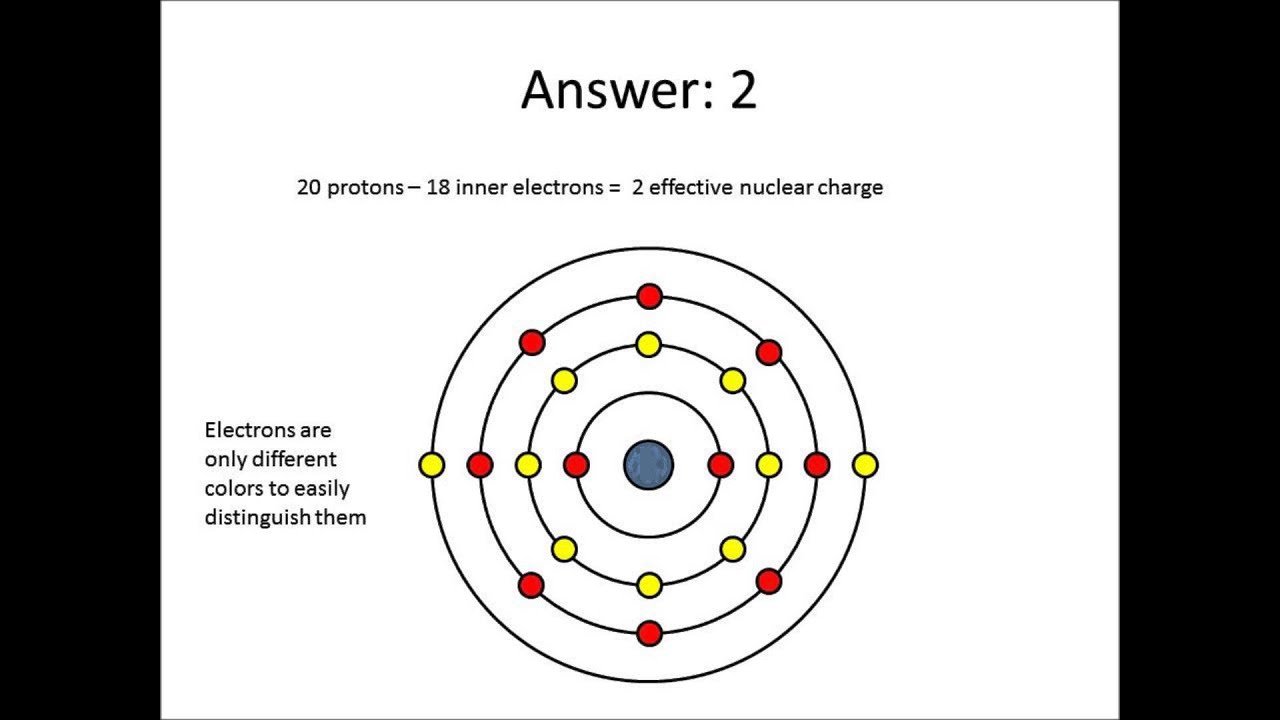
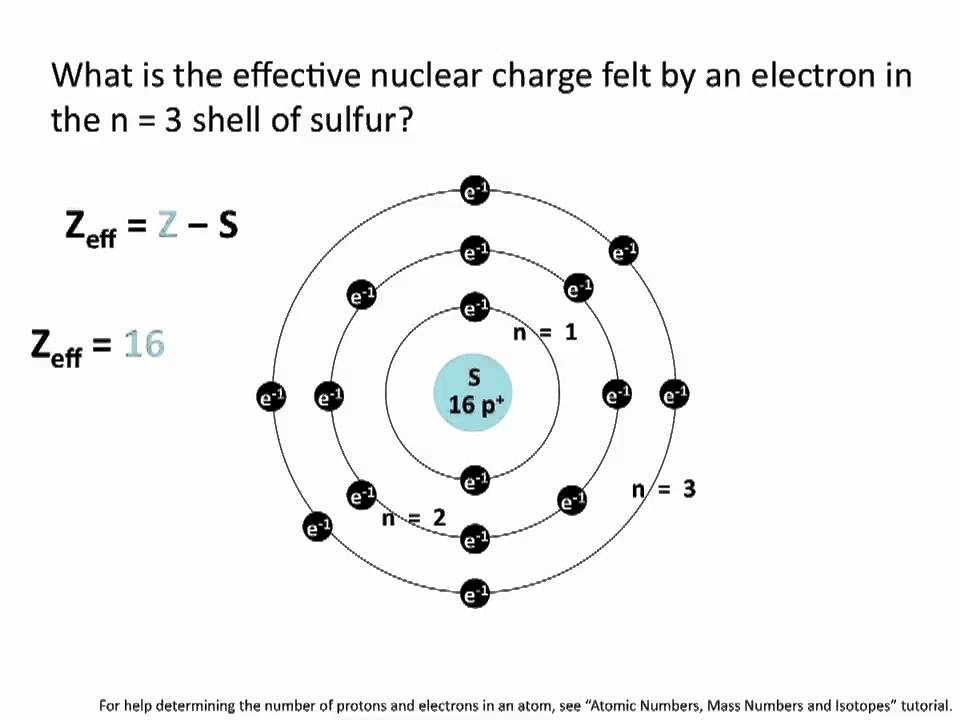
How Many Electrons Does An Atom Have
In order to find how many electrons an atom has, you simply look at the atomic number. The number of electrons = the atomic number. Chlorine for example has an atomic number of 17. That means it has 17 electrons.
Whether or not chlorine will lose or gain electrons depends upon how these 17 electrons are configured around the nucleus.
Mnemonics Will Remember The Cathode In Chemistry
In addition to the CCD mnemonic, there are other mnemonics to help identify the cathode in chemistry:
- AnOx Red Cat represents oxidation at the anode and reduction at the cathode.
- The words cathode and reduction both contain the letter c.;The reduction occurs at the cathode.
- It may be useful to associate the cat in the cation as an acceptor and an in the anion as a donor.
You May Like: How Many Valence Electrons Does Ccl4 Have
Learn What Charge Means In Science
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In the context of chemistry and physics, charge usually refers to electric charge, which is a conserved property of certain subatomic particles that determines their electromagnetic interaction. Charge is a physical property that causes matter to experience a force within an electromagnetic field. Electric charges may be positive or negative in nature. If no net electric charge is present, the matter is considered to be neutral or uncharged. Like charges repel each other. Dissimilar charges attract each other.
In physics, the term “charge” may also refer to color charge in the field of quantum chromodynamics. In general, charge refers to a generator of continuous symmetry in a system.
Do An Activity To Show That Electrons And Protons Attract Each Other
Students can see evidence of the charges of protons and electrons by doing an activity with static electricity.
Note: When two materials are rubbed together in a static electricity activity, one material tends to lose electrons while the other material tends to gain electron. In this activity, human skin tends to lose electrons while the plastic bag, made of polyethylene, tends to gain electrons.
Also Check: Cf4 Lewis Dot Structure
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Difference Between Formal Charge And Oxidation State
Often people confuse the concepts of formal charge and oxidation state. Although both these concepts probe into electron distribution, their perspectives are different, and therefore, the results are different too. It is essential to keep in mind the subtle difference between these concepts. In case of formal charge, we assume that electrons present in a bond equally distribute between both atoms. Thus, following this concept, and the formula which arises from it, we come to a value known as formal charge. However, for the oxidation state, we look into the differences in electronegativity of the two atoms. Thus, the atom having a greater tendency to attract electrons gets an advantage over the bond. Therefore, these concepts are fundamentally different, and one should not mix them up.
You May Like: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
What Is Electric Charge
In simple terms, electric charge is a physical property of matter it causes matter to experience a force when it is in an electromagnetic field. There are two types of electric charges:
Neutrons are subatomic particles with no charge. Charges which are the same repel each other, and charges which are different attract each other.
If something contains the same number of protons and electrons, it is said to be neutral overall. Something with more protons has a net positive charge and, as such, something with more electrons has a net negative charge.
What Is A Formal Charge
A Formal charge is also known as a Fake Charge. Its a theoretical charge over an individual atom of an ion as the real charge over a polyatomic molecule or ion is distributed on an;ion as a whole and not over a single atom. The formal charge of an atom of a polyatomic molecule or ion is defined below.
Read Also: Angle Addition Postulate Practice
The Role Of Charge In Electric Current
Electric current is the flow of electric charge through an object, which produces no net loss or gain of electric charge. The most common charge carriers are the positively charged proton and the negatively charged electron. The movement of any of these charged particles constitutes an electric current. In many situations, it suffices to speak of the conventional current without regard to whether it is carried by positive charges moving in the direction of the conventional current or by negative charges moving in the opposite direction. This macroscopic viewpoint is an approximation that simplifies electromagnetic concepts and calculations.
At the opposite extreme, if one looks at the microscopic situation, one sees there are many ways of carrying an electric current, including: a flow of electrons; a flow of electron holes that act like positive particles; and both negative and positive particles flowing in opposite directions in an electrolyticsolution or a plasma.
Beware that, in the common and important case of metallic wires, the direction of the conventional current is opposite to the drift velocity of the actual charge carriers; i.e., the electrons. This is a source of confusion for beginners.
|
- t }}^ }}I\,\mathrm t}
where I is the net outward current through a closed surface and q is the electric charge contained within the volume defined by the surface.
Formal Charge = Should Has
Definitely faster, right?
This shortcut is guaranteed to save precious seconds on your exam IF AND ONLY IF you understand how to apply it.
But when you understand it youll be able to solve formal charge in your head, in under 8 seconds per atom.
Lets make sure you understand this shortcut
Should = the number of valence electrons that a neutral atom SHOULD have.
Has = the number of electrons an atom HAS directly attached, touching the atom in question.
Lone pairs represent 2 electrons sitting on the atom so that Has = 2
Each bond only counts for a single electron since the second electron in the bond is touching the other atom.
Want to see this shortcut brought to life? See my formal charge video below
When you first study formal charge it helps to draw out the Lewis Structure for every molecule in question. As you get more comfortable with this topic youll be able to pick out bonds on skeletal bond-line drawings.
For now, lets stick with the basics , and look at a handful of examples utilizing the checklist shared in the Lewis Structures video below. This is a must-learn checklist so if you didnt watch yet, review this video:
As you work through Lewis Structures it helps to have a periodic table handy. Since we are working on saving you time though, your most efficient results will involve you memorizing the following 10 atoms. As you memorize their order and location, pay particular attention to these trends:
Lets apply the shortcut:
What about Nitrate?
Don’t Miss: Hawkes Learning Systems Prealgebra And Introductory Algebra Answers
Summary Valency Vs Charge
Valency gives the reactivity of an atom while charge describes how an atom has reacted. In summary, the key difference between valency and charge is that valency indicates the ability of a chemical element to combine with another chemical element, whereas charge indicates the number of electrons a chemical element gains or removes.
Reference:
1. Helmenstine, Anne Marie. What Is Valence or Valency? ThoughtCo, Mar. 21, 2019, Available here.
Image Courtesy:
2. Ions;By Jkwchui Own work via Commons Wikimedia
What Is The Valence Shell Of An Atom
The valence shell is the outermost shell of electrons surrounding an atom. The number of electrons in this shell are important for determining how the atom will react and what the charge of the ion could become.
Many of the elements you think about most often in biology and chemistry class need eight electrons in their valence shell in order to be stable. This is referred to as the .
Say you know that some atom has 10 electrons . How many would be in the valence shell? First you take away two from 10 since the first ring has 2 elections. This leaves eight electrons. That means in the valence shell there are eight electrons and that the valence shell is full.
If the valence shell is full then nothing will happen. The atom will not ionize. As a result there will be no charge on the atom.
In this example, you have neon . Neon has a full valence shell and thus does not have a charge. So what happens when the valence shell isn’t full?
Read Also: Age Problems Algebra
Difference Between Valency And Charge
July 10, 2019 Posted by Madhu
The key difference between valency and charge is that valency indicates the ability of a chemical element to combine with another chemical element, whereas charge indicates the number of electrons gained or removed by a chemical element.
Valency and charge are closely related terms as both these terms describe the reactivity of a chemical element. Valency is the combining power of an element, especially as measured by the number of hydrogen atoms it can displace or combine with. On the other hand, a charge of an atom is the number of protons minus the number of electrons in an atom.
What Is The Difference Between Valency And Charge
Valency indicates the reactivity of an atom, while charge indicates how an atom has reacted. So, the key difference between valency and charge is that valency indicates the ability of a chemical element to combine with another chemical element, whereas charge indicates the number of electrons either gained or removed by a chemical element.
Moreover, the value for valency has no plus or minus signs, while the charge has plus sign if the ion has formed by removing electrons and has the minus sign if the atom has gained electrons.
The below infographic summarizes the difference between valency and charge.
You May Like: Kendall Hunt Chemistry Answers