Some Important Concentration Terms
- Mole fraction: It is the ratio of the moles of any substance present in the solution to the total moles of the solution. Mathematically, it is given as follows:where nA is the moles of component A and nB is the moles of component B.
- Molarity: It is the concentration of any substance present in the solution. In other words, it is moles of solute per unit volume of solution in litres. Mathematically, it can be given as follows:
- Molality: It is the moles of solute dissolved in the given amount of solvent. Mathematically, it can be represented as follows:
- Normality: It is defined as the number of equivalents of solute dissolved in one litre volume of solution. Mathematically, it can be represented as follows:
Fundamental Concepts In Organic Chemistry
There are some important fundamental concepts that we need to understand carefully. These basic concepts are very useful for dealing with the organic reactions in later chapters.
- Nucleophiles: A nucleophile is a reagent that is either negatively charged or carry lone pair of electrons. These reagents attract the protons or positive charge towards themselves.
- Electrophiles: These are the reagents that are positively charged and require electrons to stabilize it.
- Inductive Effect: Inductive effect is the effect in which two different atoms of different electronegativities are bonded together. In this case, the bonded electrons are shifted towards the more electronegative atom and thus results in the polar covalent bond. When this shifting of electrons is towards the carbon then it is known as +I effect and when it is away from carbon, then it is -I effect. The relative inductive effect series based on the strength of -I effect is as follows:NH3+ > NO2 > SO2R > CN > SO3H > CHO > CO > COOH > -F > COCl > -CONH2 > Cl > Br > I > OR > -OH > -NR2 > NH2 > C6H5 > CH=CH2 > H
- Electromeric Effect: In this effect, the complete transfer of electrons takes place to one of the atoms which are directly bonded with the multiple bonds. This effect is only shown in the multiple bonds. It is temporary and becomes active only at the time of the reagent attack.
How To Prepare For States Of Matter
-
This chapter is the beginning of chemistry. This chapter is one of the most important chapters of the complete chemistry syllabus. Its concepts, laws, numerical and graphs all are important both for basic foundation of chemistry and for scoring good marks in the examination.
-
Read this chapter carefully, as it has all the basic concepts like the mole concept, stoichiometry, molarity, normality, etc.
-
This chapter is the foundation stone of the whole of the chemistry syllabus.
-
Rest this chapter is very simple, just be regular and be consistent in your numerical practice.
You May Like: Draw The Lewis Structure For Ccl4.
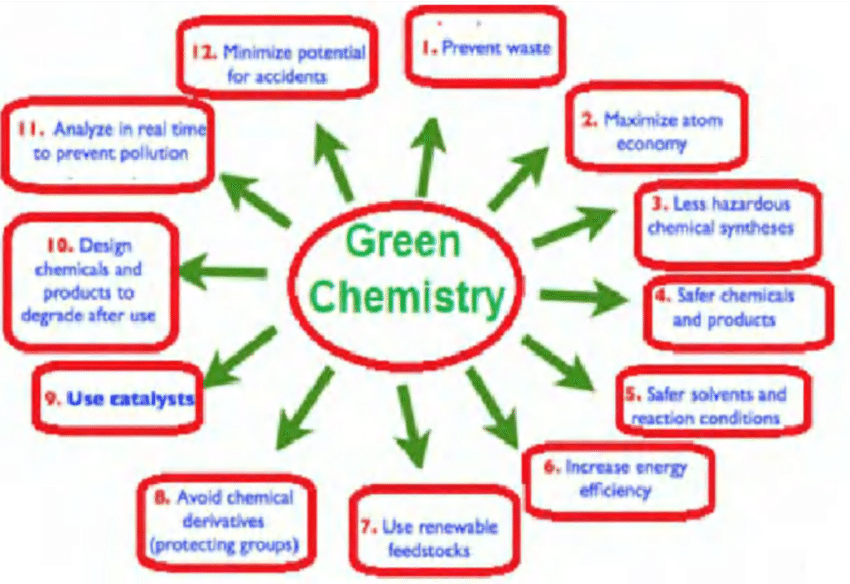
Definition Of Green Chemistry
Green chemistry is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances. Green chemistry applies across the life cycle of a chemical product, including its design, manufacture, use, and ultimate disposal. Green chemistry is also known as sustainable chemistry.
Green chemistry:
- Prevents pollution at the molecular level
- Is a philosophy that applies to all areas of chemistry, not a single discipline of chemistry
- Applies innovative scientific solutions to real-world environmental problems
- Results in because it prevents the generation of pollution
- Reduces the negative impacts of chemical products and processes on human health and the environment
- Lessens and sometimes eliminates hazard from existing products and processes
- Designs chemical products and processes to reduce their intrinsic hazards
What Is The Future Of Chemistry
Chemistryis the science that studies and manipulates matter. We human beings are gettingpretty good at it, , but we arefar from an ideal position in which we can easily make any molecule or compoundat will in a matter of minutes.
That isprobably the future of chemical synthesis, being able to shape any compound atwill in a matter of minutes, without relying on long term challenging synthesis projects. Furthermore, the possibilities ofsynthetic chemistry are literally endless: there will always be room for makinga chemical even better, or finding a molecule that works even better for agiven task.
Another keyaspect of the chemistry of the future will be reaching true fullsustainability. Chemistry will be one of the main branches of science to solvethe problem of energy.
Also,chemistry, as the central science, will be responsible to helping technologyand interdisciplinary science in general to develop smoothly.
You May Like: Who Is The Biological Father Of Paris Jackson
Essential Basic Chemistry Concepts Explained
Learning the basic chemistry concepts, in which an entire chemical education process is based on, can be overwhelming for beginners.
One of the reasons is the vast amount of information that there is out there.
For that reason, I decided to go ahead and explain here 15 important basic chemistry concepts, which hopefully will get you in a better shape for learning chemistry. These are clearly explained in most of the chemistry textbooks in our review.
In case you are starting to learn organic chemistry, we have also published an overview of the most important concepts that you will need, and a further review comparing SN1 and SN2 reactions.
We will start off with an introduction to basic chemistry: background on the most basic definitions of chemistry, a bit of history, and highlighting why and how chemistry is important. The basic units in chemistry will be defined: atoms, molecules, subatomic particles. Then, we will discuss them from a beginner point of view, and formulate them in the format of questions.
Therefore, we aim this article to people that are unfamiliar with chemistry or with science in general. If you are a teacher, you might want to redirect your students here.
Disclaimer: This is not intended to be a comprehensive description of each concept, but rather an introduction to each of them: chemistry basics for beginners. We cite and include sources that we consider useful for expanding them further.
So withoutfurther ado, lets dive in!
A Comprehensive Course In Basic Fundamental Concepts Of Chemistry Covering Everything For Future Exams In Chemistry
Best SellerCreated by Mohammad AbualrubLast updated 7/2018EnglishWhat Will I Learn?
Requirements
- There are no essential course requirements, just a desire to learn more about Chemistry and a willingness to make the necessary effort.
- This course is very simple and no need for any techniques to be understood.
Description
Hi!!!
Are you one of the high school students: Chemist student, Pharmacy student, biology student, Nursing student or Engineering student and you have problems in studying General Chemistry 101???
Do you Like Chemistry but you dont know how to study the basics in Chemistry???
Are you suffering from understanding the basics of Chemistry which makes the General Chemistry Exam as a nightmare for you???
Do you want to be a master in Chemistry and pass the Chemistry exam getting a high score easily???
Whatever the reason you have for thinking about studying chemistry , Whether you were Chemistry student, Pharmacy student, Biology student, Nursing student or Engineering student, this course will help you to understand the essential basics of Chemistry.
This course will help you in covering everything you will need to know as you prepare for possible future exams. It doesnt matter how much, or how little, prior knowledge of Chemistry youve got as this course will take you through all the necessary stages.
***************************************************************************************
Have a look at some awesome reviews about this course!!!
06:53:50
Don’t Miss: Formal Charge Of Cf4
Everything In The Universe That Has Some Mass And Occupy Some Space Is Known As Matter Matter Exists In Three Different Physical Forms Ie Solid Liquid And Gas
|
Property |
|
|
Intermediate |
Maximum |
At the macroscopic level, the matter can be classified into two categories i.e, mixtures and pure compounds as shown in the figure.
Mixtures are those substances in which two or more components are mixed. Mixtures are further classified as homogeneous and heterogeneous mixtures. Homogeneous mixtures are the one in which components are present in a fixed ratio and the properties of this kind of mixture are the same throughout, for example, solution of sugar in water. But heterogeneous mixtures are those in which the components are not mixed in a definite ratio and properties of the mixture vary at different positions of the mixture, for example, sand in water.
Tetravalency Of Carbon Shapes Of Organic Compounds
Also Read: Tetravalency of Carbon
Recommended Reading: Geometry Assignment Find The Length Indicated Answer Key
What Is The Periodic Table
The periodic table is a list or arrangement of all known chemical elements. These are organized in a way that it allows grouping elements with similar atomic structure, and therefore, similar properties. The main criteria for this order is the arrangement by increasing atomic number, which is the number of particles of that elements nucleus . Its invention is attributed to the Russian chemist Dimitri Mendeleev, and in 2019 we celebrate the 150 anniversary of his original report in 1869.
What Are Acids And Bases
Following the original definition by Arrhenius, , an acid is a compound that is able to release a hydrogen cation, or proton . For example, molecules of hydrochloric acid get ionized in solution giving a proton to water, through an acid-base equilibrium:
HCl + H2O H3O+ + Cl
HCl in water gives rise to hydronium cations and chloride anions. This is classical acid-base equilibrium.
On the other hand, bases, such as sodium hydroxide , can catch protons from water, giving rise to hydroxide anions.
NaOH +H2O HO + Na+
Whereas the Arrhenius acid-base model is very illustrative, it has its limitations, and other models are used to describe more advanced acid-base theories. The most important ones are the Brønsted-Lowry theory , and the Lewis theory.
Accordingto the Lewis theory, an acid is asubstance that accepts a lone pair of electrons, and a base is a substance thatdonates a lone pair of electrons. This accounts for acid-base equilibria whichcannot be explained by Arrhenius or Brønsted-Lowry theories, such as thebasicity of ammonia in water:
:NH3 + H2O ) NH4+ +:OH
Relative acidity or basicity of solutions or mixtures is measured using a logarithmic scale called the pH scale. It generally goes from 0, most acidic , through pH = 7 , up to 14, most basic . Nevertheless, compounds more basic and acidic than those do exist, pH = 014 is definitely not a closed range. Examples of common solutions or mixtures of different pH are shown in the scale below.
Also Check: Fsa Algebra 1 Eoc Review Packet Functions And Modeling Answers
What Types Of Chemical Compounds Are There
There are three basic types of chemical compounds, and should be briefly introduced in this post about basic chemistry concepts. All of them are the result of bonding atoms together. The difference is in the nature of the forces that hold together those atoms.
- In molecules , which are neutral compounds of individual nature, atoms are glued together by covalent bonds. Covalent bonds generally occur between two non-metal atoms, which share pairs of electrons, or bonding pairs.
- In ionic compounds, atoms are in ionic form and are held together by ionic forces, giving rise to large networks of oppositely charged ions. Ionic bonds occur between metals and non-metals.
- When extended networks of atoms are formed between one or more types of metal atoms, we are talking about metallic bonds.
What Is Some Basic Concepts In Chemistry

‘Some basic concepts of chemistry’ is the most fundamental chapter of complete chemistry. It gives information about the atomic number and mass number of elements. In any chemical reaction, it is important for us to know about the number of reactants that will consume and the products that will produce, thus for estimating all these calculations, we use the laws of chemical combinations. In this chapter, there are other important topics as well as the mole concept, stoichiometry, molarity, normality, etc.
There are some important real life application based on the chapter of some basic concepts in chemistry.
- In cooking, it is always necessary to know the exact amount of ingredients to be add in the food to get the best and tasty dish. This calculation of ingredients in stoichiometry.
- Saturn planet is much larger than earth but still, it is less dense. The specific gravity of Saturn is even less than 1.
- The GPS system that we use for finding out the way is related to stoichiometry. All these signals come from satellites. The stoichiometry helps to calculate the fuel and its components of reactions in the complete journey of satellites.
Read Also: How To Find Biological Grandparents Uk
What Is Some Basic Principles Of Organic Chemistry
This is the beginning of organic chemistry. In this chapter, you study the basic concepts like IUPAC nomenclature, isomerism, inductive effect, resonance effect. There are some reagents as well like nucleophile and electrophile that comes into picture only during the time of organic reactions. In isomerism, you will study the detailed analysis of isomerism and its various types. There are many organic compounds that have the same chemical formula but they differ in their physical properties. Besides, you will also study the benzene compounds and their substituents.
For this chapter, there are various real-life applications that we observe in our daily life. Some of them are mentioned below.
- For petrochemical products, most of the petrochemical products are hydrocarbons and their derivatives natural gas, kerosene, gasoline, etc.
- Resonance of molecules is the most stable structure of molecules and most stable structure is its middle form among all the resonance structures. This same phenomenon is used in swings.
Organic Chemistry Some Basic Principles And Techniques
Also, Read: Important Question on Organic Chemistry
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
Nomenclature Of Organic Compounds
Also, Read: IUPAC Nomenclature
Overview Of The Chapter
In this chapter, you will study the basic concepts related to organic chemistry. First, you will learn the reason due to which carbon is able to form a large number of compounds. Besides, you will learn the rules to name these organic compounds, isomerism, resonance effect, inductive effect, etc. Besides, you will also learn important tips and the best book to prepare this in the most efficient way.
Tetravalence of Carbon
Tetravalency is one of the major reason due to which carbon is able to form many compounds. While the formation of a compound, carbon forms the hybrid orbitals and because of these hybrid orbitals, these molecules have a definite shape. Other than sigma bond, carbon forms bond as well. In this type of bonding, the hybrid orbitals are not involved but they are formed by p-orbitals. In bond, the parallel orientation of p-orbitals occurs on adjacent carbon atoms.
Structural representations of Organic Compounds
-
Complete, condensed and Bond-line structural formulas:In 2-dimensional geometry, carbon compound structures are represented in three ways, i.e, complete structural formula, condensed structural formula and bond-line structural formula.
Bond-line structural formula: In this type of structural representation, carbon and hydrogen atoms are not shown only the dashes or bonds are shown. Other atoms or groups like chlorine, oxygen, etc. shown.
On the basis of the structures, these organic compounds are classified as follows:
Also Check: How To Do Elimination In Math
Green Chemistry’s Roots In The Pollution Prevention Act Of 1990
To stop creating pollution in the first place became America’s official policy in 1990 with the Federal Pollution Prevention Act .
The law defines as any practice that:
- Reduces the amount of any hazardous substance, pollutant, or contaminant entering any waste stream or otherwise released into the environment prior to recycling, treatment, or disposal.
- Reduces the hazards to public health and the environment associated with the release of such substances, pollutants, or contaminants.
The term “source reduction” includes:
- Modifications to equipment or technology
- Modifications to process or procedures
- Modifications, reformulation or redesign of products
- Substitution of raw materials
- Improvements in housekeeping, maintenance, training, or inventory control
Section 2 of the Pollution Prevention Act establishes a pollution prevention hierarchy, saying:
- The Congress hereby declares it to be the national policy of the United States that pollution should be prevented or reduced at the source whenever feasible
- Pollution that cannot be prevented should be recycled in an environmentally safe manner, whenever feasible
- Pollution that cannot be prevented or recycled should be treated in an environmentally safe manner whenever feasible an
- Disposal or other release into the environment should be employed only as a last resort and should be conducted in an environmentally safe manner.
For those who are creating and using green chemistry, the hierarchy looks like this:
What Does Chemistry Mean
As an introduction to chemistry, it is the branch of science that studies matter and change. First, chemistry deals with the study of the composition and the properties of matter . Then, chemistry deals with change, or how these substances evolve when submitted to certain conditions, or how one substance changes or reacts while interacting with a different substance. The definition of chemistry cant be made shorter, since it covers basically everything!
Also Check: Geometry Segment Addition Postulate Worksheet