What Makes Water Such An Ideal Solvent For Reactions
The water molecule H2O features two hydrogen atoms covalently bound to oxygen.
But this is not a balanced covalent bond.
Oxygen is highly electronegative and pulls on the electrons between itself and hydrogen. This concentrates negativity around the oxygen atom, leaving both hydrogen atoms partially exposed with a partial positive charge.
To really understand this we need just ONE oxygen and ONE hydrogen. In most reactions, the oxygen is ultimately de-protonated so that just one hydrogen is removed.
So lets assume the other hydrogen is just extra baggage,
a backpack.
If we call that extra hydrogen a backpack and turn it into a circle, does the reaction still proceed as expected?
Chances Are Theres More Than One Way To Derive Your Product
When I took my first weekly Organic Chemistry 2 quiz, I inadvertently set a trend of scoring top of the class, but it was a fluke. I didnt realize we had weekly quizzes
We were asked to outline a step by step procedure to separate two similar molecules with different functional groups. The process involved a series of reactions to prepare one molecule for extraction.
I tried!
I remembered that there were about six steps, but I could only confidently answer four. Id already bombed, then aced Orgo 1, and didnt want to do that again!
No matter what I did, I couldnt come up with the other steps. So instead, I crossed out my four and a half steps and wrote a detailed step by step procedure for carrying out fractional distillation.
My TA very reluctantly gave me full credit with an amused warning.No one else came close.
Am I asking you to outsmart the question?
Not quite.
What If It Wasnt That Easy
What if youre asked to start with an alkyne?
We cant go from alkyne to alcohol directly, since the enol product would immediately tautomerize to a ketone or aldehyde.
This is where we introduce many options:> If you reduce the alkyne to an alkene, you may use one of the following as already discussed above:
HOWEVER, in this example were starting with a 3-carbon chain yet ending with a 4-carbon chain.
4. Do I know of a reaction that produces an intermediate to the above product?Yes! Terminal alkynes easily undergo chain elongation via SN2.
We’ll start with an acid/base reaction to deprotonate the terminal alkyne forming a good nucleophile.
We need to elongate the chain by just one carbon. Lets give this methyl group a good leaving group to facilitate a quick SN2 reaction my go-to is Bromine, but you can also use Chlorine or Iodine.
Now that we have a carbon chain of desired length, lets carry out the acid catalyzed hydration.
But wait, the product is an enol, not a ketone!!
The next step will happen automatically. So while you dont have to show a reagent simply draw KET over the reaction arrow for Keto Enol Tautomerization.
And there we have it!
Read Also: How To Psychologically Break Someone
Yup Nothing Changed Its Just The Oh Reacting With A Backpack
Now what if I turn that backpack into something else,
For example an ANY alkyl group represented by the letter R.
Same mechanism, but since the product has an OR instead of an OH the product is an ether instead of an alcohol.
Lets try another backpack using a methyl group:
And an isopropyl group:
Do you see how oxygen still reacts the same way?
Oxygen’s electrons still attack the carbocation, and the one hydrogen is still removed in the final deprotonation step. This happens regardless of the other group or backpack attached to the carbon atom.
Program That Simulates Basic Reactions In Organic Chemistry
To explain – a friend and I have been working on a program that would allow you to draw organic molecules, indicate the conditions, and predict the products. This would be primarily aimed at basic, first year undergrad organic chemistry synthesis, etc).
We’ve realized that it is probably worthwhile to figure out if there are similar existing programs out there. From what we’ve read there doesn’t seem to be something that meets what we’re planning – everything we’ve found seems to be aimed at professional/higher level chemists, not assisting a beginner.
Basically it comes down to a couple main points:
Are you aware of similar software?
Do you think that this sort of software would be used .
If/when we have a working version, would users here be interested in using/testing it?
I apologize if this doesn’t meet the guidelines/standards for questions here, I was kind of iffy about asking it.
You May Like: What Is The Difference Between Math And Mathematics
Title: Linking The Neural Machine Translation And The Prediction Of Organic Chemistry Reactions
Abstract: Finding the main product of a chemical reaction is one of the importantproblems of organic chemistry. This paper describes a method of applying aneural machine translation model to the prediction of organic chemicalreactions. In order to translate ‘reactants and reagents’ to ‘products’, agated recurrent unit based sequence-to-sequence model and a parser to generateinput tokens for model from reaction SMILES strings were built. Training setsare composed of reactions from the patent databases, and reactions manuallygenerated applying the elementary reactions in an organic chemistry textbook ofWade. The trained models were tested by examples and problems in the textbook.The prediction process does not need manual encoding of rules to predict products, hence it only needs sufficient trainingreaction sets to learn new types of reactions.
Using This Logic Heres The Shortcut:
Thats it, thats your final product.
Lets see how this works for acid catalyzed hydration with water:
Now the same reaction with an alcohol:
Lets try this with oxymercuration-demercuration:
And now lets try alkoxymercuration which is the same reaction as above but with alcohol, ie: oxygen carrying a carbon chain backpack.
This tutorial is just the first step to learning and understand organic chemistry concepts and reactions. Dont let the semester take you by surprise forcing you to play catch-up. Click below to download EVERY Leah4sci Orgo Cheat Sheet in a single full color PDF download. Youll also get access to tutorials, study tips and tricks, and Upcoming LIVE Workshop announcements!
Don’t Miss: Why Is Geography Important In The Study Of History
Use Study Partners For Homework & Tests
Youll be astonished at what you learn when you have to explain something to others. Dont ever feel like you need to tackle organic chemistry by yourself. Getting together to do your work with your fellow students is a fantastic idea. Not only can others help you with concepts youre struggling with you can also get a stronger grasp on the material you already know by explaining it to someone else.
To conclude, theres no magic to doing well in the organic chemistry. It involves discipline, doing problems, focusing on learning concepts, and staying on top of the material.
Did you find this post useful? Share your thoughts in the comment section.
Remember organic chemistry makes up a big chunk of the JEE Advanced question paper. Its important to be thorough with this section to do well in the exams!
Easy Way To Remember Organic Reactions And Solve Conversion Questions Easily
- Post Author:Chemist
- Post published:May 7, 2020
Organic conversions are easy if you are well aware about organic compounds and their reactions. It is quite a challenge to remember all the reactions of all chapters so, summarizing important reactions of different chapters in a flow chart is very effective.
- It captures the most essential reactions of the chapters in one diagram.
- Easy for revision.
- A visual representation has more retention in memory.
Stuck the conversion charts on the wall of your reading room, so you can see reactions frequently and memorize easily.
Contents
Conversion Chart of Aliphatic Compounds
- Organic conversion process need not be a single step every time. You always start with initial reactants and move to intermediate steps , between your initial reactants and the final product. The most important thing that you should master is the art of guessing the Intermediates. Once you guess the correct intermediate you can write the reaction that takes you from reactant to intermediates and then the reaction that takes you from intermediate to the product. For this you have to practice many questions. Generally asked questions in any exam are repeated, so practice of old questions is effective.
- You can use conversion chart to make the conversion problems easier. Look at the final compound you want to obtain and initial compound and see the various intermediate compounds formed between reactant and product step by step.
Sample Question :
Stepping up:
Also Check: What Does Biological Sister Mean
How To Lean Organic Chemistry Reaction Mechanisms In A Easy Way Can You Please Tell Me Some Reference Books And Note Books Are Better Or Some Links About This Matter
Hello Aspirant,
Organic chemistry is totally based on the learning of concepts. First of all you need to learn the name reactions, name reactions are just like the formulas in mathematics which can solve your multiple problems. In Organic Chemistry with similar name reactions a number of products can be made many times. You need to go through the mechanism and understand the mechanism so that you can understand the whole thing.
I would prefer you to stick with the basic one i.e. NCERT Textbooks Class XI & XII. There is no need to study hard level books now. First clear your concept with NCERTs.
After that you can go for some reference books like :-
- Organic Chemistry by O.P. Tandon
- Organic Chemistry by M.S. Chauhan
- Organic Chemistry by Morrison and Boyd
For more details refer to the link given below :-
I hope this information helps you.
Good Luck!!
Struggling With Organic Chem Reactions
babytomato said:I’m about halfway done with watching Chad’s ochem videos on coursesaver. I’ve been getting down the alkene/alkane reactions and a few others but it’s starting to get confusing because there are so many reactions/rules/exceptions. Was it easy for you guys to memorize the reactions? Is there a better way to remember them?
IdleKoala said:The best thing I can recommend is to do practice problems. I learn the best from doing problems, getting them wrong, and learning why. So I would start doing practice problems ASAP. Also, DAT Bootcamp has a list of all the most important organic chemistry reactions. I studied that list daily and it really helped me. Best of luck.
IdleKoala said:Thanks, i’ve been very blessed! The list was good for reaction problems definitely, but there are a lot of other questions in organic chemistry besides reactions. Yes this is something that supersizes a lot of people, most people think that its going to be all calculation problems, but in my experience it was about 1/3 calculation problems and 2/3 conceptual. This was good for me, cause I suck at doing math quickly. Bootcamp exams do a really good of replicating this and prepares you really well.
Recommended Reading: How To Study Ap Human Geography
Build A Strong Foundation
Students tend to skip the beginning of the lecture and early chapters of material because its background or really easy and jump into the material they dont understand. This is a recipe for self-defeat! Organic chemistry is like a pyramid the top will collapse without a strong base. Even if you think you have no time, go back to the introduction and early chapters and spend some time reviewing this material. You will likely be surprised how much better you will grasp the hard material once you completely understand the easy bit!
Types Of Reactions In Organic Chemistry
Hope it will help
Organic chemistry involves,
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions.
Plz mark it as brainlist
Answer:
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions.
Recommended Reading: What Is An Example Of Geography
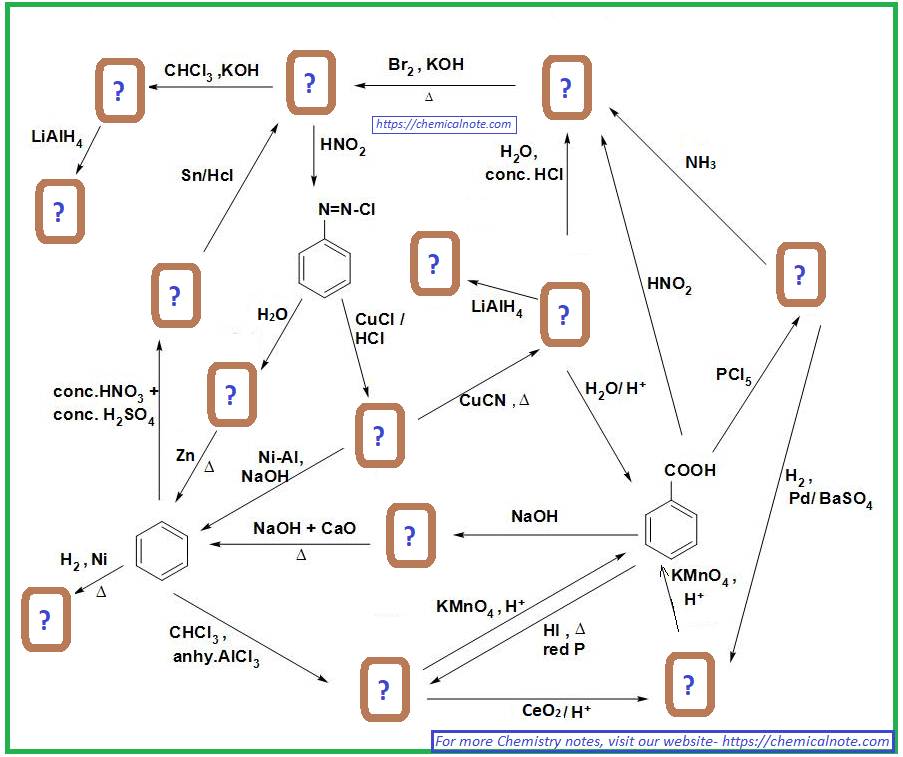
Aromatic Substitution Practice Problems
Show how each compound can be synthesized from benzene and any other organic or inorganic reagents.
The order of reactions is very important! So, before every step, consider the ortho , para , or meta directing effect of the current group on the aromatic ring.
Devise a synthesis of each of the following compounds using an arene diazonium salt. They all require more than one step and you may select the desired regioisomer when needed.
Just Recognize That The More Reactions You Learn The More Options You Have For Creating A Single Functional Group
If you’re stuck on a certain pathway or cant fully describe the steps,Ask yourself, Is there another way to create the same functional group?
Think back to the many alcohol formation reactions we discussed above. If you forgot one option, simply use another.
Here are a few interesting patterns and alternates to consider.
> Chain Elongation Go-To reactions
- Use alkynes in Orgo 1
- Use grignards or condensation in Orgo 2
> Adding carboxylic acids or carbonyls
- Alcohol -> oxidation
- Oxidative cleavage using KMnO4 or O3
- Grignard and CO2
> Moving Reactivity so that you can start/react at a different portion of the molecule compared to the current location of the active group.
Active groups include leaving groups, pi bonds, nucleophilic centers susceptible to attack and more.
Here are some of my favorite moving the reactive location’ tricks:
- Move the Pi bond intermediate then apply a .
- No pi bond? Radical halogenation to introduce a leaving group and THEN eliminate.
These are just SOME of the tricks you can utilize.
Don’t Miss: Does Elton John Have Biological Children
How To Name A Compound
Organic Reactions: An Introduction
These are the four “prototypical” organic chemistry reactions, though several others which can be categorized as one of these are generally referred to by other names. Look at these reactions and ask yourself this question for each: what bonds are broken, and what bonds are formed.
At least 80% of the reactions students in organic chemistry fall into one of these four categories. The sooner you can get into the habit of recognizing bond formation and breakage the better off you will be. A fifth reaction is also discussed: rearrangement reactions.
You May Like: What Does N Stand For In Chemistry
Synthesis Of Compound A
Synthesis of compound a
Toluene, on reaction with bromine molecule in the presence of ferric bromide, yields p-bromotoluene.
The treatment of p-bromotoluene with butyryl chloride in the presence of substitutes the butyryl group on the meta position of p-bromotoluene .
The methyl group can be converted into the alcohol functional group by transforming it into alkyl bromide and then treating it by a base.
Solution For Problem 84 Chapter 8
Organic Chemistry | 11th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Organic Chemistry | 11th Edition
Write a mechanism for the following reaction. practice problem 8.4 OH cat. H2SO4 H2O What general conditions would you use to ensure a good yield of the product? What general conditions would you use to carry out the reverse reaction, i.e., the dehydration of cyclohexanol to produce cyclohexene? What product would you expect to obtain from the acid-catalyzed hydration of 1-methylcyclohexene? Explain your answer.
THESE ARE CH. 9 & CH. 10 REVIEW NOTES THESE ARE THE NOTES FROM THE SILDES FOR CH. 11 THESE ARE THE KEY NOTES FROM THURSDAYS 1/18/17 THESE ARE THE NOTES FROM SI SESSION
ISBN: 9781118133576
Other solutions
You May Like: Algebra Two Step Equation Practice
Maximizing Partial Credit On Your Exam
Gaining bonus points on exams is one thing, but heres the best part:
The average synthesis question is worth anywhere from 10-30 points.And the average professor WILL give partial credit.So, if you can only remember four of five steps, DO NOT leave it blank to receive zero points!
Instead, write the four steps and add in as much relevant information as possible.Then VERY CONFIDENTLY fake the fifth step.
Do not write Magic! .
Make up something that appears to be just a careless mistake. Your professor will be impressed by your work and hopefully give you an 80% for the question.
This also applies to reagents!
If you only remember the steps, but dont remember which reagents will get you there, start by drawing out the molecules:
A > B > C
This has two benefits:
Of course if Reagents are giving you trouble with this, here’s a video on Memorizing Organic Chemistry Reagents.
And if you cant remember them, INVENT SOMETHING rather than leave it blank. Try to use a reagent that has the groups youre adding.
For example, if you forget that an alkyne will react with HgSO4 in H2SO4 to yield a ketone,
Are they correct?Not quite!Both will cleave the alkyne.