What Is Meant By Concentration Of A Solution
In an aqueous solution, two parts exist, namely solute and solvent. They are the two basic solution concentration terms that you need to know. We always need to keep an account of the amount of solute in the solution. The amount of solute in the solvent is what is called the concentration of a solution. In chemistry, we define concentration of solution as the amount of solute in a solvent. When a solution has more solute in it, we call it a concentrated solution. Whereas when the solution has more solvent in it, we call it a dilute solution. Now that you understand the concept of what is concentration of solution let’s move on to the different methods of expressing concentration.
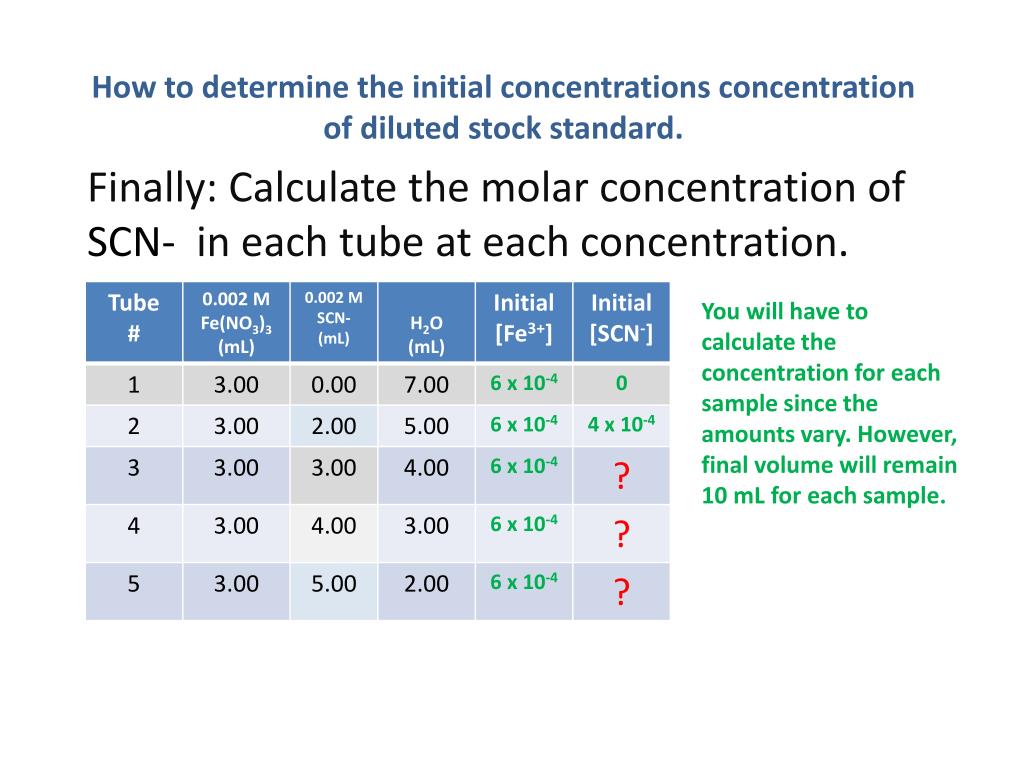
The image shows a solution from the most dilute solution to the most concentrated solution.
Plot Of Concentration Versus Time For A Zero
Recall that the rate of a chemical reaction is defined in terms of the change in concentration of a reactant per change in time. This can be expressed as follows:
\text = -\frac}} = \text
=-\text
This is the integrated rate law for a zero-order reaction. Note that this equation has the form \text=\text. Therefore, a plot of versus t will always yield a straight line with a slope of -\text.
How To Calculate Ph In Chemistry
- The Albert Team
Attention: This post was written a few years ago and may not reflect the latest changes in the AP® program. We are gradually updating these posts and will remove this disclaimer when this post is updated. Thank you for your patience!
Abstract: pH is a unit of measurement often used in fundamental chemistry concepts. How to Calculate pH explains its categories the scientific mathematics and role pH has in our lives.
Read Also: How To Login To Imagine Math
General Equations For Finding Concentration
The following shows a generalized version of the steps shown above or “concentration formula.”
Given moles of solute and liters of solution, molarity is calculated by:
Given the amount of solute in grams, the following equation gives concentration:
Given the amount of solute in milligrams, the following equation gives concentration:
Related Articles
How To Calculate Poh

pOH is determined by the concentration of OH, . This can be calculated by the following equation:
pOH=-log or pOH=log \right) }
Conversely, the hydroxide concentration can be found by a given pOH. The can be calculated by the following equation.
\left =^
pOH is a different way of describing acidity and basicity, so be careful not to mix it up with pH. The descriptions for solutions based on the pOH scale are given in Table 2.
Table 3. The pOH Scale
| Basic | |
| 7 | Greater than 7 |
The determination of the concentration of hydroxide ions and pOH will be later used to show the relationship between pH and pOH.
Also Check: Michael Jackson Kids Biological Parents
Examples About Molarity Values
- If 1 dm3 aqueous solution contains 0.45 mol of HCl, molarity of HCl is 0.45 mol dm-3. Otherwise, we can describe this is as, 0.45 mol of HCl is dissolved 1 dm3 of the total solution.
- If 10 dm3 aqueous solution contains 0.98 mol of HNO3, molarity of HNO3 is 0.098 mol dm-3.
Sometimes we use ” to represent concentration of solute. As an example, to represent concentration of aqueous HCl, we can use
Example: = 0.1 mol dm-3
What Is Concentration Of Solution
Everyone talks about the concentration of solutions. They may also talk about the concentration of coffee or tea. Everyone has a particular view of what is meant by the concentration of a solution. You must have noticed that whenever you make coffee if you add a lot of powder, you will end up with a concentrated drink, whereas if you add little, it will result in a dilute solution. Therefore, it is essential that you understand what is the concentration of the solution. In this chapter, we will learn about what is meant by the concentration of a solution we will also see how to find the concentration of a solution and the different methods of expressing concentration of solution.
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers
Using The Method Of Initial Rates To Determine Reaction Order Experimentally
2\ \text_\text_\rightarrow 4\ \text_+\text_
The balanced chemical equation for the decomposition of dinitrogen pentoxide is given above. Since there is only one reactant, the rate law for this reaction has the general form:
\text= \text^}
In order to determine the overall order of the reaction, we need to determine the value of the exponent m. To do this, we can measure an initial concentration of N2O5 in a flask, and record the rate at which the N2O5 decomposes. We can then run the reaction a second time, but with a different initial concentration of N2O5. We then measure the new rate at which the N2O5 decomposes. By comparing these rates, it is possible for us to find the order of the decomposition reaction.
Calculate Concentration Of Aqueous Na2co3 In Mol Dm
In a Na2CO3 solution, concentration of Na+ is found that 0.008 mol dm-3. Calculate the concentration of Na2CO3 in mol dm-3.
Na2CO3 hydrolysis is occurred as followings in thhe water.
Na2CO3â 2Na+ + CO32-
According to the stoicheometry ratio, one Na2CO3 mol gives two Na+ moles.
So concentration of Na2CO3 is half of the concentration of Na+ ions.
Concetration of Na2CO3 = 0.008 / 2
Concetration of Na2CO3 = 0.004 mol dm-3
Your Questions
Read Also: What Is The Molecular Geometry Of Ccl4
We Dissolve 2 Moles Of Salt In 1dm3 Of Water To Make A Solution Find The Molar Concentration
This is a straight forward question. You are provided two required data to find the concentration.
- Amount of moles = 2 moles
- Volume of the solution = 1 dm3
Then substitue the values to concentration equation.
- Concentration = amount / volume
- Concentration of salt = 2 mol / 1 dm3
- Concentration of salt = 2 mol dm-3
How To Calculate Molarity
Recommended Reading: What Does Abiotic Mean In Biology
Calculating Molarity Given Moles And Volume
If there are 10.0 grams of NaCl dissolved in water to produce 2.0 L of solution, what is the molarity of this solution?
First, we must convert the mass of NaCl in grams into moles. We do this by dividing by the molecular weight of NaCl .
10.0 \text \times \frac}} = 0.17 \text
Then, we divide the number of moles by the total solution volume to get concentration.
\text_\text=\frac_\text}}
\text_\text=\frac}}
\text_\text = 0.1 \text
The NaCl solution is a 0.1 M solution.
Molar Solution Concentration Calculator
Here is the simple online molar concentration calculator to calculate the molarity substance which is expressed as mol/L. It is defined as the number of moles of solute dissolved in a liter of solution and formula is defined as x . Molarity calculation is used in teaching, laboratory, study and research. In the below molar solution concentration calculator enter the mass, volume and molecular weight and click calculate to find the molarity.
Formula:
Where,
You May Like: What Is Figure Ground Perception Psychology
Molarity Of Distilled Water
Now we are going to discuss about how rto calculate molarity of water in mol dm-3. Take the density of water as 1000 g dm-3.
First calculate the mass of water in 1 dm3
mass = volume * density
mass = 1 dm3 * 1000 g dm-3
mass = 1000 g
Next, find how many moles are in 1dm3 of distilled water.
Number of moles = mass / molecular weight
Number of moles = 1000 g / 18 g mol-1
Moles = 55.56 mol
Finally, calculate the concentration
Concentration = dissolved moles / total volume
Concentration = 55.56 mol / 1 dm3
Concentration of water = 55.56 mol dm-3
S Of Calculating Solution Concentration
California State Standard: Students know how to calculate the concentration of a solute in terms of grams per liter, molarity, parts per million, and percent composition.
Grams per liter represent the mass of solute divided by the volume of solution, in liters. This measure of concentration is most often used when discussing the solubility of a solid in solution.
Molarity describes the concentration of a solution in moles of solute divided by liters of solution. Masses of solute must first be converted to moles using the molar mass of the solute. This is the most widely used unit for concentration when preparing solutions in chemistry and biology. The units of molarity, mol/L, are usually represented by a scripted capital M.
Parts per million , is a ratio of parts of solute to one million parts of solution, and is usually applied to very dilute solutions. It is often found in reports of concentration of water contaminants. To calculate parts per million, divide the mass of the solute by the total mass of the solution. This number is then multiplied by 106 and expressed as parts per million . In dilute water solutions, we can assume that 1 mL of water-based solution has a mass of 1 gram, so 1 liter of solution has a mass of 1000 grams.
Read Also: The Angle Addition Postulate Answer Key With Work
Integrated Rate Law For Second
For a reaction that is second-order overall, and first-order in two reactants, A and B, our rate law is given by:
\text=-\frac}}=-\frac}}=\text
There are two possible scenarios here. The first is that the initial concentrations of A and B are equal, which simplifies things greatly. In this case, we can say that =, and the rate law simplifies to:
\text=\text^2
This is the standard form for second-order rate law, and the integrated rate law will be the same as above. However, in the case where _0\neq _0, the integrated rate law will take the form:
\text\frac=\text\text
In this more complicated instance, a plot of \text\frac versus t will yield a straight line with a slope of \text.
Calculating Mass Given Molality
We can also use molality to find the amount of a substance in a solution. For example, how much acetic acid, in mL, is needed to make a 3.0 m solution containing 25.0 g of KCN?
First, we must convert the sample of KCN from grams to moles:
\text = 25.0 \text \times = 0.38 \text
The moles of KCN can then be used to find the kg of acetic acid. We multiply the moles by the reciprocal of the given molality so that our units appropriately cancel. The result is the desired mass of acetic acid that we need to make our 3 m solution:
0.38 \text \times = 0.12\text
Once we have the mass of acetic acid in kg, we convert from kg to grams: 0.12 kg is equal to 120 g. Next, we use the density of acetic acid to convert to the requested volume in mL. We must multiply by the reciprocal of the density to accomplish this:
120.0 \text \times = 114.0 \text
Therefore, we require 114 mL of acetic acid to make a 3.0 m solution that contains 25.0 g of KCN.
Molarity vs. molality: In this lesson, you will learn how molarity and molality differ.
Read Also: Kendall Hunt Geometry Answer Key
Calculating Volume Given Molarity And Moles
We can also calculate the volume required to meet a specific mass in grams given the molarity of the solution. This is useful with particular solutes that cannot be easily massed with a balance. For example, diborane is a useful reactant in organic synthesis, but is also highly toxic and flammable. Diborane is safer to use and transport if dissolved in tetrahydrofuran .
How many milliliters of a 3.0 M solution of BH3-THF are required to receive 4.0 g of BH3?
First we must convert grams of BH3 to moles by dividing the mass by the molecular weight.
\frac\text_3 }\text_3} = 0.29 \text\text_3
Once we know we need to achieve 0.29 moles of BH3, we can use this and the given molarity to calculate the volume needed to reach 4.0 g.
\text_}=\frac_}}}
3.0 \text = \frac_3} }
\text = 0.1 \text
Now that we know that there are 4.0 g of BH3 present in 0.1 L, we know that we need 100 mL of solution to obtain 4.0 g of BH3.
How To Calculate Concentration In Ppm
When youre working with solutions in chemistry, expressing the concentration of one component relative to the whole mixture is essential.
A lot of the time, youll be able to use more everyday measures like percent to convey the strength of the mixture, but in other cases youll need less common units like parts per million or PPM.
This is basically what it sounds like: The number of parts of the thing youre interested in for every 1 million parts of the solution. But its important to know how to perform a basic PPM calculation if youre going to communicate with other scientists.
Recommended Reading: Michael Jackson Kids Biological
How To Calculate Molality Of A Solution
Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same.
Calculate Molality: moles solute per kilogram solvent
symbol: m
m = moles / kilogram
Example: What is the molality of a solution of 3 grams of KCl in 250 ml of water?
First, determine how many moles are present in 3 grams of KCl. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. Then add them together to get the grams per mole for KCl.
- K = 39.1 g/mol
- KCl = 39.1 + 35.5 = 74.6 g/mol
For 3 grams of KCl, the number of moles is:
* 3 grams = 3 / 74.6 = 0.040 moles
Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water , but you also have 3 grams of solute, so the total mass of the solution is closer to 253 grams than 250. Using 2 significant figures, it’s the same thing. If you have more precise measurements, don’t forget to include the mass of solute in your calculation!
- 250 g = 0.25 kg
- m = 0.040 moles / 0.25 kg = 0.16 m KCl
How To Find The Concentration Of A Solution Using Different Methods
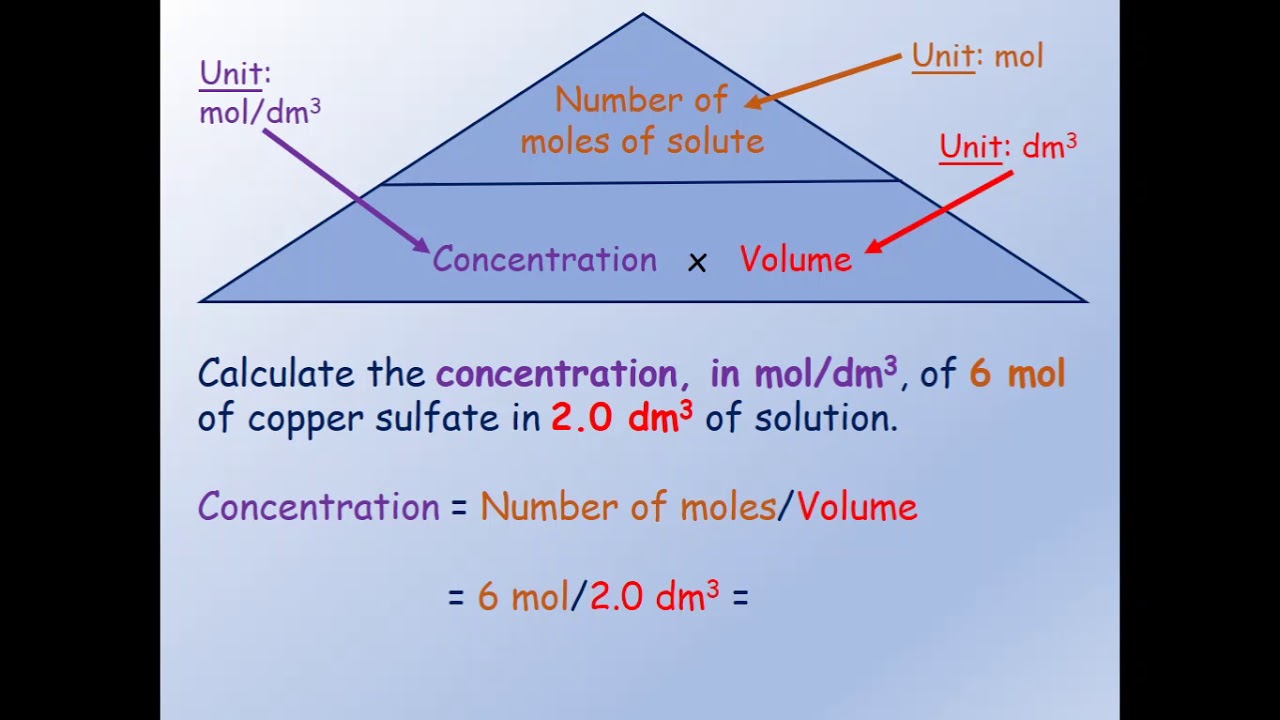
There are various methods of expressing concentration of a solution. You will usually see Chemists working with the number of moles. Pharmacists will use percentage concentrations instead of the number of moles. Hence it is important to understand all the methods of expressing concentration of solutions.
The concentration of solution formula is given as follows.
Cor S = \
We will also see other methods on how to calculate concentration of a solution based on the different methods of expressing concentrations.
Don’t Miss: Figure And Ground Psychology
Properties Of Mass Concentration
S Per Million Calculations
Recall that, in general, concentration tells you how much solute is present in a solution.
concentration = amount of solute ÷amount of solution
A concentration in parts per million may refer to the mass of solute present in the volume of solution or it may refer to the mass of solute present in a mass of solution .
In SI units, w/w concentration would be given in kilograms of solute per kilograms of solution. So, a 1 part per million solution would be 1 kg of solute per 1 million kilograms of solution. And these masses are just too large to be useful in Chemistry laboratory. But we can divide the masses of solute and solution by 1 million to arrive at more useful units:
| 1 ppm |
ppm = mass of solute ÷ mass of solution
ppm = mass of solute ÷ mass of solution
You should practice rearranging the equations above in order to find mass of solute, volume of solution or mass of solution:
- To calculate mass of solute:
- To calculate volume of soution
- To calculate mass of soution
mass of solute = ppm × volume of solution
mass of solute = ppm × volume of solution
mass of solute = ppm × mass of solution
mass of solute = ppm × mass of solution
volume = mass of solute ÷ ppm
volume = mass of solute ÷ ppm
mass of solution = mass of solute ÷ ppm
mass of solution = mass of solute ÷ ppm
Also Check: What Is An Inertial Frame Of Reference In Physics
How To Calculate Dilutions
You dilute a solution whenever you add solvent to a solution. Adding solvent results in a solution of lower concentration. You can calculate the concentration of a solution following a dilution by applying this equation:
MiVi = MfVf
where M is molarity, V is volume, and the subscripts i and f refer to the initial and final values.
Example:How many milliliters of 5.5 M NaOH are needed to prepare 300 mL of 1.2 M NaOH?
Solution:5.5 M x V1 = 1.2 M x 0.3 LV1 = 1.2 M x 0.3 L / 5.5 MV1 = 0.065 LV1 = 65 mL
So, to prepare the 1.2 M NaOH solution, you pour 65 mL of 5.5 M NaOH into your container and add water to get 300 mL final volume