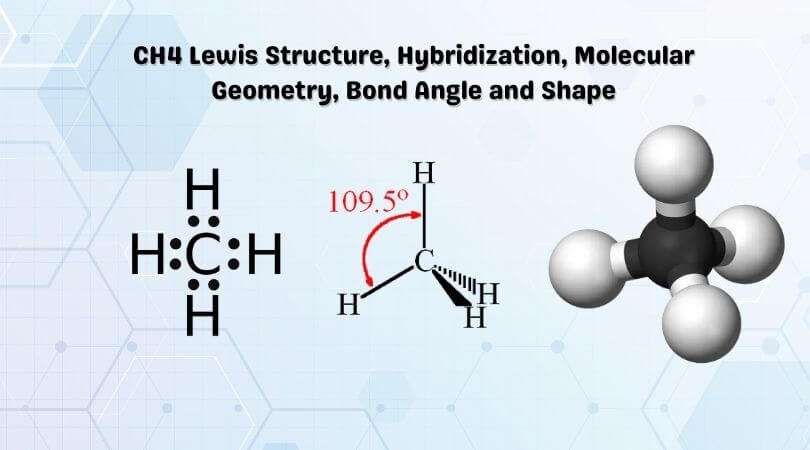
Ch4 Lewis Structure Molecular Geometry And Hybridization
Methane or CH4 is a naturally occurring gas and relatively abundant on the Earth, making it an economically efficient fuel. As it releases more light and heat on burning, it is preferred more than coal, fossil fuel, or gasoline for energy production.
It is one reason why overproduction of methane has made it a considerate greenhouse gas where it is affecting the temperature and climate system of the Earth.
The Lewis structure is a pictorial representation of how many valence electrons are present in an atom.
Moreover, the diagram also helps with determining how the bond formation is taking place between the atoms to form a molecule, ultimately a compound.
The Lewis diagram is drawn by showing valence electrons in the form of dots drawn around the atom and lines predicting the bond formation.
These lines also determine whether a single, double, or triple bond has been formed helping with predicting the hybridization of the central atom.
What Are The Electron And Molecular Geometry Of Ch4
The molecular geometry of CH4 is Tetrahedral. The carbon central atom is located in the center of the tetrahedron, while the four hydrogens atoms are located on the vertices. These atoms repel each other in a way that the final shape of CH4 appears like Tetrahedral.
In CH4, the carbon central atom has no lone pair and is attached to the four hydrogen atoms with the help of a single covalent bond. So, there are four regions of electron density around the carbon central atom.
The electron pair around the carbon central atom will repel each other and tried to go far from each other, they will take the position where repulsion becomes minimum between them.
According to the VSEPR theory, in most of the cases, the central atom with four regions of density adopt a tetrahedral structure because repulsion is minimum in electron pairs at this position.
In the above figure, the red lines outline the tetrahedron and the black lineshows the bonded pair or electron pair formed between carbon and hydrogen. The carbon atom is located in the center of the tetrahedron, while the four hydrogens atoms are located on the vertices.
So, there are four bonding pairs formed in CH4 molecule, hence, according to the VSEPR theory, they will repel each other, as a result, all corners atoms spread out as much as they can and takes the place where the repulsion is minimum and stability is much better.
AXN notation for CH4 molecule:
So, the AXN notation for the CH4 molecule becomes AX4N0 or AX4.
Nh4+ Lewis Structure Molecular Geometry And Hybridization
NH3 is the chemical formula of Ammonia. A positively charged polyatomic ion of Ammonium or NH4+ comes into existence when an Ammonia atom goes through the process of protonation, that is, it loses one of its electrons and becomes positively charged.
A protonated Ammonium ion or NH4+ is made up of Nitrogen and Hydrogen. The ion is the by-product of a chemical reaction between a proton donor and Ammonia, which is as follows:
NH3 + H+ > NH4+
Recommended Reading: What Is Phylogeny In Biology
Key Points To Consider When Drawing The Ch4 Lewis Structure
A three-step approach for drawing the CH4 Lewis structure can be used. The first step is to sketch the Lewis structure of the CH4 molecule, to add valence electron around the carbon atom the second step is to valence electron to the hydrogen atom, and the final step is to combine the step1 and step2 to get the CH4 Lewis Structure.
The CH4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH4 molecule. The geometry of the CH4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory , which states that molecules will choose a CH4 geometrical shape in which the electrons have from one another.
Finally, you must add their bond polarities to compute the strength of the C-H bond . The carbon-hydrogen bonds in methane, for example, are polarised toward the more electronegative carbon, and because both bonds have the same size, their sum is zero due to the CH4 molecules bond dipole moment, and the CH4 molecule is classified as a nonpolar molecule.
The molecule of methane is tilted at 109 degrees and has a difference in electronegativity values between hydrogen and carbon atoms, with hydrogens pull being roughly equal to carbons. As a result, it has no dipole moment indefinitely. The CH4 molecule has no dipole moment due to an equal charge distribution of negative and positive charges.
The Electronic Configuration Of The Valence Electrons Of Carbon Is 2s22p2
In our review of atomic orbitals, we saw that the orbital configuration of the valence electrons of carbon is 2s22p2 as shown below:
Since the 2s orbital is lower in energy than 2p, its filled first. That means that there are two electrons in the 2s orbital, and a single electron in two of the three 2p orbitals. Theres also an empty 2p orbital.
Don’t Miss: What Is Precipitation In Geography
Tetrahedral Carbons: Not A Popular Idea In 1874
The eminent German chemist Hermann Kolbe had this to say:
For his part, vant Hoff flew to Stockholm on his Pegasus to receive the first Nobel Prize in Chemistry in 1901.
Incontrovertible proof for the tetrahedral arrangement of bonds around the carbon atom came in 1913 when Bragg determined the structure of diamond using X-ray crystallography and found it to be a tetrahedral network of carbon atoms with C-C-C bond angles of 109.5°.
Why Is The Molecular Geometry Of Ch4 Is Same As Its Electron Geometry
The molecular geometry of CH4 is tetrahedral and its electron geometry is also tetrahedral because as per VSEPR theory, molecular shape considers only bond pairs or atoms while electron geometry considers bonded atoms as well as lone pairs present on the central atom.
According to the lewis structure of CH4, the central atom doesnt contain any lone pair on it.
Hence, only bonded atoms are used to determine the geometry of CH4.
Therefore, Molecular geometry of CH4 = Electron geometry of CH4
Read Also: What Does Finite Mean In Math
C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
Hydrocarbons form an essential and inseparable portion of the science of chemistry. Be it petroleum, crude oil, or natural gas, the majority of hydrocarbons are found naturally in these fossil fuels. Apart from this, we can find them in synthetic polymers and other man-made plastic materials.
They are organic in nature and as the name suggests, they are formed of only carbon and hydrogen. Sometimes, it also creates compounds with other varieties like sulfur, nitrogen, and so on.
Although these are some of the simplest organic compounds we can come across, they have a varied range and differ in several physical and chemical properties.
The Geometrical Structure Of Methane
The single-molecule of methane is tetrahedral with no lone pairs on any atom. This behavior is explained with the help of the Valence Shell Electron Pair Repulsion theory.
This theory is used to predict the geometrical structure of a molecule along with the reason for such a shape.
For the methane molecule, this theory says as there exists no distortion in the structure of CH4, it is an ideal bent-shaped molecule or tetrahedron having a bond angle of 109.5° between hydrogen-carbon-hydrogen atoms .
Due to the symmetrical shape of the bonds formed in the CH4 molecule, the charges on its atoms are equally distributed and no polarization takes place ie the Methane molecule is a nonpolar molecule.
For better understanding, you can refer to the article written on the polarity of CH4.
The distortion from the ideal bond angle within a molecule occurs because of the presence of lone pairs and bond length between the central atom and the side atoms.
From the Lewis structure, it can be understood that an equal number of electron sharing is taking place between the carbon atom and four hydrogen atoms altogether.
It is the reason why the structure of methane is highly stable in nature.
You May Like: What Does Inhibition Mean In Biology
What Is The Dot Structure Of Hydrogen Sulfide
On both sides of the central sulfur atom in the H2S Lewis structure, there are two hydrogen atoms.The molecule bends due to the existence of two unbonded pairs of electrons.The molecule is slightly polar because sulfur is more electronegative than hydrogen.In the case of H2S, the vectorial sum of the bond dipole moments results in a non-zero total dipole moment. As a result, dipole-dipole interactions are observed in hydrogen sulfide.
What Is Clf3 Molecular Geometry
CLF3 has a T-shaped molecular geometry and trigonal bipyramidal electron geometry. According to the ClF3 Lewis structure, this molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound.
Umair has been working at Whatsinsight since 2020 as a content writer. He has a Masters degree in Materials Science.
Don’t Miss: What Does Abiotic Mean In Biology
Methane Lewis Dot Structure Molecular Geometry Electron Geometry Polar Or Nonpolar Bond Angle
Home > CH4 lewis structure and its molecular geometry
Methane is a colorless and odorless gas formed from one atom of carbon and four atoms of hydrogen having the chemical formula CH4. It is the simplest of saturated hydrocarbons. It is used for the generation of electricity by burning as a fuel in steam generator or gas turbine.
In this article, we will discuss Methane lewis dot structure, molecular geometry, electron geometry, hybridization, polar or nonpolar, its bond angle, etc.
Methane is mainly used as a fuel for turbines, water heaters, ovens, etc. It is also used in industrial chemical processes to prepare other chemicals.
Properties of Methane
Lets see how to draw the lewis structure of CH4 by following some simple steps-
Simple Steps For Drawing The Lewis Dot Structure For Ch4
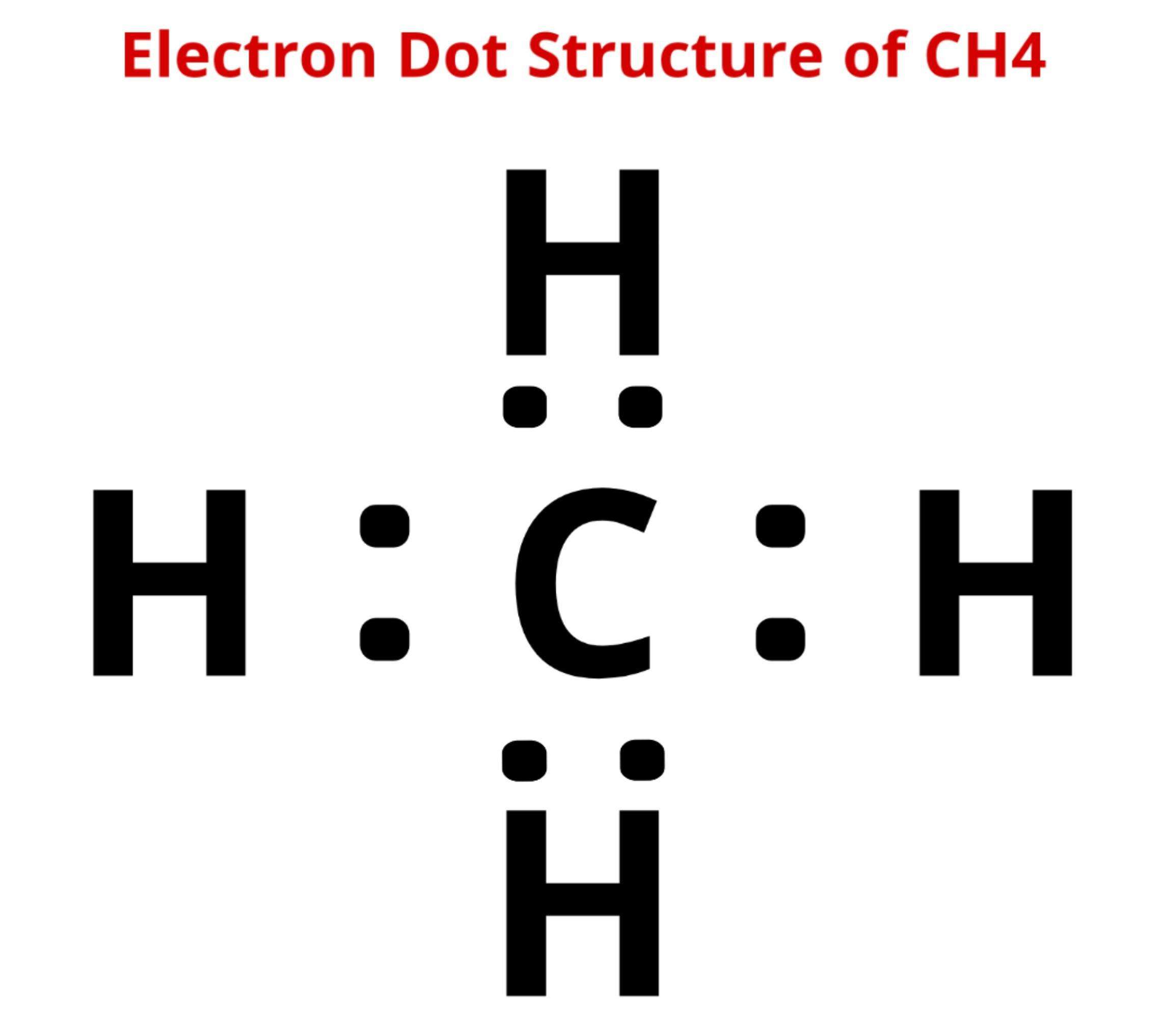
1. Count total valence electron in CH4
In the first step, we have to find how many valence electrons are there in CH4, so that we can distribute them around central and terminal atoms with the goal of completing their octet shell.
You have two ways to find out the valence electron for a particular atom, either by looking at their periodic group or by writing their electronic configuration. We will use the method of the periodic group for finding the valence electron in CH4.
The carbon atom belongs to Group 4A or 14A in the periodic table, hence, it has a 4 valence electron in its outermost shell whereas the hydrogen atom belongs to Group 1A, hence, it has only 1 valence electron in its outermost shell.
Total valence electron in Carbon = 4
Total valence electron in Hydrogen = 1
Total valence electron available for drawing the CH4 lewis structure = 4 + 1*4 = 8 valence electrons
2. Find the least electronegative atom and placed it at center
Whenever hydrogen is present in any molecule then it doesnt matter which atom is less or more electronegative, hydrogen always goes outside in a lewis diagram and it needs only two electrons to complete its outer shell.
So, place all Hydrogen atoms outside in the lewis diagram and the Carbon atom at the central position.
3. Connect each outer atom to the central atom with a single bond
In this step, we will simply connect each outer atom to the central atom with the help of a single bond.
= 0 valence electrons
Also Check: How To Calculate Precision Physics
Maybe Methane Is Square Planar
Alright, you say. If all C-H bonds are of equal lengths and angles, why cant CH4 have the structure below, where all the bond angles are 90° and CH4 is flat, in the plane of the page. .
This was in fact the majority opinion for the arrangement of bonds around carbon until about 1880. Extremely brilliant chemists such as Berzelius went to their graves having no reason to doubt that methane was anything but flat.
However, we now know this to be wrong. Why?
What Are The Electron And Molecular Geometry Of Cf4
The molecular geometry of CF4 is Tetrahedral. Since all four fluorine atoms are attached to a carbon atom, and no lone pair is present on the central atom. According to the VSEPR theory, any molecule that has four bonded atoms linked with a central atom and no lone pair present on the central atom acquires the tetrahedral geometry.
Also, all fluorine atoms bonded to carbon atoms lie at the corners of the tetrahedron with a 109.5º bond angle between them.
The electron geometry of CF4 is also tetrahedral.
Lets see how to find the electron and molecular geometry of the CF4 molecule:-
Don’t Miss: What Is An Experimental Study In Psychology
How To Calculate The Formal Charge On The Carbon Atom In Ch4 Lewis Structure
The formal charge on the CH4 molecules carbon central atom often corresponds to the actual charge on that carbon central atom. In the following computation, the formal charge will be calculated on the central carbon atom of the CH4 Lewis dot structure.
To calculate the formal charge on the central carbon atom of CH4 molecule by using the following formula:
The formal charge on the carbon atom of CH4 molecule= L.E 1/2)
V.E = Valence electron in carbon atom of CH4 molecule
L.E = Lone pairs of an electron in the carbon atom of the CH4 molecule.
B.E = Bond pair electron in C atom of CH4 molecule
calculation of formal charge on carbon atom in CH4 molecule
The carbon core atom of the CH4 molecule has four valence electrons, zero lone pair electrons, and eight bonding electrons. Put these values for the carbon atom in the formula above.
Formal charge on carbon atom of CH4 molecule = ) =0
In the Lewis structure of CH4, the formal charge on the central carbon atom is zero.
The Hybridization Of Nh4
The concept of Hybridization decrees that atomic orbits fuse with one another to form new degenerated hybrid orbitals, which influence bonding properties and molecular geometry of the atoms of an element.
It can be considered as an extension of the valence bond concept and lays its foundation on the molecular and quantum mechanics of an atom.
These new orbitals may have different shapes, energies, etc. when compared to the previous ones. Hybridization brings about changes in the orbital arrangement of an atom as well.
Such a structure arises from the need for a refined geometry of atoms necessary for electrons to pair up and thus, form different chemical bonds, as inducted by the valence bond theory.
These hybrid orbitals, formed by the hybridization of an atom, are helpful in the explanation and understanding of an atoms molecular geometry, its atomic bond properties, and the position in the atomic space.
In most common scenarios, atomic orbitals with similar energy combine to form hybrid orbitals.
While the exchange between atomic orbits of different atoms leads to the creation of molecular orbits, hybridization of an atom is assumed to be a combination of different atomic orbits, overlaying one another in different fractions.
Atomic orbits of comparable levels of energy participate in forming hybrid orbitals. This process can also involve half-filled and fully filled orbitals as well, provided that the level of energy remains similar.
Recommended Reading: Chapter 7 Test Form 2a Algebra 2
Overview: Ch4 Electron And Molecular Geometry
According to the VSEPR theory, CH4 possesses a tetrahedral molecular geometry and a CH4-like electron geometry. Because the centre atom, carbon, has four C-H bonds with the four hydrogen atoms surrounding it. The H-C-H bond generates a 109-degree angle in tetrahedral geometry. The CH4 molecule has a tetrahedral shape because it contains four hydrogen atoms.
There are four C-H bonds at the top of the tetrahedral geometry. After linking the four hydrogens in the tetrahedral form, it maintains the tetrahedral-like structure. In the CH4 tetrahedral geometry, the C-H bonds are enclosed.
The centre carbon atom of CH4 has no lone pairs of electrons, resulting in tetrahedral electron geometry. However, the molecular geometry of CH4 is tetrahedral in nature. Its the CH4 molecules asymmetrical geometry. As a result, the CH4 molecule is nonpolar.
Key Points To Consider When Drawing The Ch4 Molecular Geometry
A three-step approach for drawing the CH4 molecular can be used. The first step is to sketch the molecular geometry of the CH4 molecule, to calculate the lone pairs of the electron in the central carbon atom the second step is to calculate the CH4 hybridization, and the third step is to give perfect notation for the CH4 molecular geometry.
The CH4 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH4 molecule. The geometry of the CH4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory and molecular hybridization theory, which states that molecules will choose a CH4 geometrical shape in which the electrons have from one another.
Finally, you must add their bond polarities to compute the strength of the C-H bond . The carbon-hydrogen bonds in the methane molecule, for example, are polarised toward the more electronegative carbon atom, and because both bonds have the same size, their sum is zero due to the CH4 molecules bond dipole moment, and the CH4 molecule is classified as a nonpolar molecule.
The molecule of methane is tilted at 109 degrees and has a difference in electronegativity values between hydrogen and carbon atoms, with hydrogens pull being roughly equal to carbons. As a result, it has no dipole moment indefinitely. The CH4 molecule has no dipole moment due to an equal charge distribution of negative and positive charges.
You May Like: What Is Transparent In Physics
Explain Co Dot Structure In Simple Words
The overall carbon to oxygen atom ratio in a CO dot structure is 1:1. By sharing three valence electrons, carbon forms three tipple bonds with oxygen. Its octet is now complete. Similarly, to complete its octet, oxygen shares three valence electrons with carbon. Oxygen also possesses a lone pair of electrons.