Pf3 Lewis Structure Molecular Geometry And Hybridization
Phosphorus trifluoride is colorless as well as an odorless gas having similar toxicity as that of carbon monoxide. It binds to the iron present in the blood hemoglobin to spread throughout the body and prevents the blood from absorbing the oxygen.
PF3 is a nucleophile where it donates a pair of electrons during a chemical reaction.
Lewis diagram is a pictorial presentation of the number of valence electrons present in an atom, which readily reacts with the valence electrons of another atom to form a bond.
The diagram is drawn with the help of eight dots around the atom, mostly in pairs. Here, the number eight has been selected as per the octet rule.
Moreover, the line represents the formation of a bond between the valence electrons of the two atoms. Counting these lines can help with determining how many bonds have been formed within a molecule.
Why The Bond Angle Of Ph3 Is Lesser That That Of Pf3
We can explain why the bond angle of $\ce$ is lesser than $\ce$ by the VSEPR theory, since lone pair lone pair repulsion is greater than lone pair bond pair repulsion. Then for $\ce$ and $\ce$, also, it is expected that the bond angle of $\ce$ will be smaller. But I found that the thing is just reverse. Why is this so? Is it an exception?
- 3$\begingroup$I don’t see how VSEPR can explain the bond angles in $\ce$ and $\ce$. Take a look at this question for the correct reason.$\endgroup$Aug 21, 2015 at 17:52
VSEPR works in the cases of $\ce$ and $\ce$. In reality, there are much more things to consider as shown in this answer. All four compounds should have bond angles of $90^\circ$ if there were no other effects present.
For both nitrogen compounds, the effects are the short $\ce$ bonds which lead to steric clash of the three substituents. Thus, the bond angle is broadened from the original, hypothetical $90^\circ$ to whereever it may seem comfortable to the molecule. Since fluorines are larger atoms than hydrogens are, the $\ce$ bond is longer than the $\ce$ bond and therefore the atoms can agree on a smaller bond angle .
The answer can not be determined using VSEPR theory. The number of lone pairs in both compounds is the same. The answer lies in electronegativity.
In $\ce$ nitrogen is more electronegative than hydrogen and therefore it will pull the electrons towards it. Therefore the electrons will be closer to each other hence more repulsion.
How Do You Determine Molecular Geometry
Steps Used to Find the Shape of the Molecule
Also Check: Apex Learning Answer Key Algebra 2 Sem 1
Why Bond Angle In Pf3 Greater Than Ph3
This is because, in the PF3 molecule, back bonding occurs.
The exchange of electrons between an atomic orbital on one atom and an antibonding orbital on another atom is known as back bonding.
Due to back bonding, higher bond pair-bond pair repulsion exists in PF3 than PH3.
Hence, the higher the repulsion between bonded pairs, the larger is the bond angle.
Also, in the PF3 molecule, fluorine is a highly electronegative atom and it fetched more electrons towards itself which also creates more repulsion, and this causes, widening the bond angle in PF3.
The approximate bond angle of PH3 is 93° and for PF3, it is 96°.
What Is The Molecular Shape Of Pf3
Based on VSEPR Theory the electron clouds on the atoms, and the lone pair of electrons around the N, will repel each other. As a result they will be pushed apart giving the PF3 molecule a trigonal pyramidal molecular geometry or shape.
Moreover, is pf5 symmetrical?
Research VSEPR theory for further info. In short. PF5 adopts a trigonal bipyramidal shape not symmetrical. It shares a similarity with the shape of SF6 in that both these shapes have an atoms in an axial plane.
What is the shape of if5?
Decision: The molecular geometry of IF5 is square pyramidal with asymmetric charge distribution on the central atom.
What is the shape of a bf4?
The molecular geometry shape of BF4- is tetrahedral. A step-by-step explanation of how to draw the BF4- Lewis Structure. As you can see here, BF4- has 32 valence electrons. Boron has 3 valence electrons, and each of the four fluorides contributes one electron to each covalent bond.
Recommended Reading: What Does Dilation Mean In Math
Why Is The Bond Angle Of Molecules With T
In a T shaped molecule based on trigonal bipyramidal electron-pair geometry, there are two lone pairs. The near-90 degree bond angles are between the axial positions and the one equatorial bond. Of course the angle between the two axial bonds is nTest 3 Recitation 14) Give the electron geometry , molecular geometry , and hybridization for XeF4. eg = tetrahedral, mg = tetrahedral, sp3 eg = trigonal pyramidal, mg trigonal pyramidal, sp3PF3 Draw the Lewis structure for PF3 in the box at the right, including lone pairs. What is the molecular The Lewis structure for PO_4^3+ is shown. What is the electron-pair geometry and the molecularChemistry I Chapter 10Determine the electron geometry and molecular geometry of the underlined carbon in CH3CN. OF2 < PF3 < PF4 Place the following in order of decreasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in
What Is The Lewis Structure Of Bf3
There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.
Is PF3 polar or non polar?
Answer = PF3 is Polar. What is polar and non-polar? Polar. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
Also Check: Girls Get Curves Geometry Takes Shape
Geometrical Structure Of Phosphorus Trifluoride Molecule
The geometrical structure of the tetra-atomic Phosphorus Trifluoride molecule is studied with the help of the Valence Shell Electron Pair Repulsion theory.
This theory explains that the bond angle between the fluorine-phosphorus-fluorine is 97°. This angle makes the structure bent where the ideal bond angle for the bent, trigonal pyramidal structure is 109.5°.
This anomaly is due to the lone pair of electrons, and the smaller size of the fluorine atom. As the lone pair repulsion is stronger than the bond pair or bond pair-lone pair repulsion, it reduces the bond angle.
There exist a lone pair of electrons on the phosphorus which does not participate in the bond formation. Because they are highly stable, their repulsion is stronger than that of the bonding pair of electrons.
When any shared pair of electrons come in the near vicinity of lone pair of electrons, as do not want to bond.
This repulsion distorts the whole structure where the effect increases to many folds because of the smaller size of fluorine and shorter atomic radius distance.
Due to its original pyramidal shape, the PF3 molecule turns out to be polar. You can also check an article related to the polarity of PF3.
What Is The Molecular Shape Of Of2
The molecular geometry of OF2 is bent and its electron geometry is tetrahedral because the presence of two lone pairs on the central atom creates repulsion with bonded pairs of electrons, as a result, all outer atoms pushes down in order to minimize the repulsion according to the VSEPR theory, and that makes
Read Also: What Is Fission In Chemistry
Pf3 Lewis Structure Formal Charge
The formal charge is defined as the charge over a particular molecule assuming that all the atoms have the same electronegativity.
F.C. = Nv Nl.p. -1/2 Nb.p.
Nv = number of electrons in the valence shell of the free atom
Nl.p = number of electrons in lone pair
Nb.p = number of electrons involved in the bond formation.
So the formal charge of the PF3 molecule is 26–6 = 2
Some Facts About Phosphorus Trifluoride
The molar mass of PF3 is 87.98 g/mol, density is 3.91 g/l. The melting point and boiling point of PF3 are 121.7 K and 171.4 K respectively. Its shows a chemical shift value at -34 in 19F NMR. PF3 is normally synthesized via the halogen exchange reaction between phosphorus trichloride and various metal fluorides such as ZnCl2 or Cacl2.
Don’t Miss: What Does Migration Mean In Biology
What Is Molecular Geometry
The study of the three-dimensional arrangement of the atoms that constitute a molecule is called Molecular geometry.
Molecular geometry gives information about the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.
In covalent molecules the bonds are of directional nature because the shared pairs of electrons remain localized in a definite space between the nuclei of the participating atoms. The space model obtained by joining the points which represent the different bonds in a covalent molecule accounts for its shape.
Why Does Ph3 Have A Lesser Bond Angle Than Pf3
Considering the formation of PF3 where three F atoms approach the central p atom along the three axes. When the 3 F atoms come at bonding distance they will suffer steric repulsion due to the lone pair of P as well as F atoms also. Hence the system will go unstable. In order to gain stabilization, the central P atom undergoes sp3 hybridization and the bond angle becomes 970.
When the 3 H atoms approach the P center in a similar fashion, it is due to the smaller size of H and larger size of the P atom 3 H atoms will not suffer any steric repulsion. Thus the system is not energies and the px, py, pz orbitals of P are directly involved in the bond formation, and no need for hybridization thereby accounting for the H-P-H bond angle being around 900.
You May Like: Why Do We Sleep Psychology
On The Geometry And Inversion Process Of Pf3+ X
· The C2v-Y form of both PF3 and PF~- shows long P-F bonds, due to the lone pair/lone electron being localized on the P nucleus. A CISD minimum could not be located for the cationic C2v-Y form. As a final remark on geometries, it should be noted that, in all cases, P-F bond distances decrease in going from B3LYP via MP2 to CISD.
Is T Shaped The Same As Trigonal Pyramidal
In chemistry, T-shaped molecular geometry describes the structures of some molecules where a central atom has three ligands. Ordinarily, three-coordinated compounds adopt trigonal planar or pyramidal geometries. The three atoms bond at 90° angles on one side of the central atom, producing the T shape.
You May Like: What Is The Basic Unit Of Chemistry
Molecules With More Than One Central Atom
The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. We take in account the geometric distribution of the terminal atoms around each central atom. For the final description, we combine the separate description of each atom. In other words, we take long chain molecules and break it down into pieces. Each piece will form a particular shape. Follow the example provided below:
Butane is C4H10. C-C-C-C is the simplified structural formula where the Hydrogens are implied to have single bonds to Carbon. You can view a better structural formula of butane at en.Wikipedia.org/wiki/File:Butane-2D-flat.png If we break down each Carbon, the central atoms, into pieces, we can determine the relative shape of each section. Let’s start with the leftmost side. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon. That means that we have 4 electron groups. By checking the geometry of molecules chart above, we have a tetrahedral shape. Now, we move on to the next Carbon. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. Again, we have 4 electron groups which result in a tetrahedral. Continuing this trend, we have another tetrahedral with single bonds attached to Hydrogen and Carbon atoms. As for the rightmost Carbon, we also have a tetrahedral where Carbon binds with one Carbon and 3 Hydrogens.
Based On What You Know About Vsepr Theory What Is
Based on the above Lewis diagram, we can see there is 1 lone pair on the central P atom. We can also see the central atom has 4 negative charge centers . In addition What are the bond angles of PF_3 and H_2Se? · You can count the number of valence electrons each atom contributes and generate a reasonable electron geometry. PHOSPHORUS TRIFLUORIDE P: 5 F: 7 From the above, PF_3 contains 5+21 = 26 valence electrons. 6 are lone pairs for each F. 6 are required to make three total sigma bonds. That accounts for \\mathbf. Therefore, one last lone pair accounts
Recommended Reading: How Does Evolutionary Psychology Explain Human Behavior
Is Hcn Straight Or Curved
Cyanide of Hydrogen
The Lewis diagram for HCN displays carbon at the centre with no lone electron pairs. The carbon and nitrogen are connected through a triple bond which counts as one electron pair. As a result, the molecule is linear and has two electron pairs. It boils at 25°C, making it a room-temperature gas.
What Is The Molecular Geometry Shape Of Pf3
Geometrical Shape To identify if the compound is polar molecules, you can check the molecular geometry that it produces. The Fluorine has seven valence electrons in the outermost shell, while the Phosphorus has 5. The geometrical shape of Phosphorus trifluoride is trigonal pyramidal, so PF3 is a polar particle.
You May Like: Cpm Algebra Chapter 7 Answers
Valence Electrons In Phosphorus And Fluorine Atom
The electrons present in the outermost shell of an atom are called valence electrons. Because they are present in the outermost shell, the hold of the nucleus is weak on them.
Moreover, uneven or unpaired electrons compel them to participate in the bond formation.
Here, it is essential to understand that a higher number of valence electrons will strengthen the ability of an atom to accept the electrons rather than donating.
So, fluorine accepts the electron whereas, the phosphorus atom tends to donate the electrons to complete their octet and reach a stable condition. The reason for the same is explained with the help of their electronic configuration.
The atomic number of phosphorus is fifteen which makes its electronic configuration 1s2 2s2 2p6 3s2 3p3. As we know, the p shell can hold a maximum of six electrons there is a scarcity of three electrons.
Whereas, on the other hand, the atomic number of fluorine is nine which makes its electronic configuration 1s2 2s2 2p5, having a scarcity of only one valence electron.
Why Is The Molecular Geometry Of Pf3 Is Trigonal Pyramid Whereas Its Electron Geometry Is Tetrahedral
This is because there is a slight difference in molecular and electron geometry. Molecular geometry takes only bonded atoms into account while calculating the shape of any molecule.
Whereas the electron geometry considers both bonded atoms and lone pairs while predicting the geometry of any molecule.
The phosphorous central has 4 regions of electron density hence, as per VSEPR theory, the electron geometry of PF3 is distorted tetrahedral.
Whereas the molecular geometry of PF3 is trigonal pyramidal that has one atom at the center and three atoms at the trigonal base corners and it is similar to a tetrahedron but it does not consider lone pair.
Properties of Phosphorous trifluoride
Read Also: How To Increase Torque Physics
How Does The Pf3 Lewis Dot Structure Obey The Octet Rule
If an atom gets more or less than 8 electrons in an outermost shell then we can say that atom violates the octet.
Phosphorous atom has five valence electrons in its outermost shell and it is capable of forming three covalent bonds with the neighboring atom to complete its octet.
Whereas the fluorine atom has 7 valence electrons and is capable of forming only one covalent bond with other atoms.
Therefore, in the PF3 lewis structure, phosphorous as a central atom forms three covalent bonds and fulfills its octet requirement whereas the fluorine atom also completes its octet by one covalent bond attached with the central atom.
All atoms in the PF3 lewis dot structure get exactly 8 electrons in their octet, hence, they are obeying the octet rule
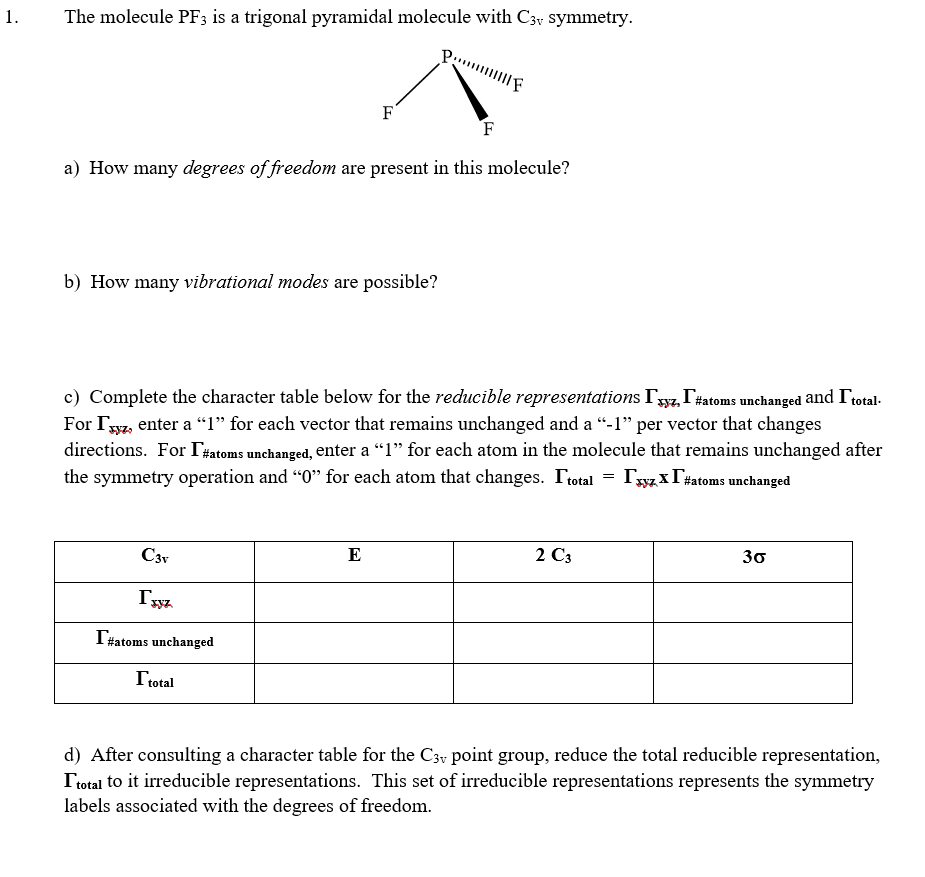
Molecular Orbitals Diagram Of Phosphorus Trifluoride Molecule
The molecular orbital diagram helps with determining how chemical bond formation is taking place. Also, it helps with figuring out how mixing and overlapping have taken place to produce four new hybrid orbitals.
The mixing and overlapping occur in the orbitals of similar energy, whereas the bonding electrons contribute to the formation of higher energy antibonding molecular orbitals.
Also Check: What Grade Is Biology Taught
What Is Hybridization
It involves the mixing of atomic orbitals having similar energy to form an equal number of mixed orbitals or hybrid orbitals and these hybrid orbitals are so oriented in space that they can overlap with suitable orbitals of the subsequent. If the orbitals are of the same energy is called equivalent hybridization and if the mixed orbitals are of different energy then it is called non- equivalent hybridization.
| sp3d3/d3sp3 | 900,720 |
In the ground state, the electronic configuration of Phosphorus is 3s23p3. We know the maximum number of electrons occupying in p orbital is 6. So here lack of electrons is 3. Now the electronic configuration of fluorine is 2s22p5.
So, to complete the octet of Phosphorus it needs 3 more electrons, and to complete the octet of Fluorine it removes one electron. So, each Fluorine gives one electron to the vacant p orbital of Phosphorus and completed its octet and after taking three electrons even Phosphorus completes its octet too.
So, a stable bond is a formation that happens via the mixing of s and three p orbitals. Phosphorus has two electrons in its 3s orbital as a lone pair and its 3p orbital is filled with six electrons . So here in hybridization one s orbital and 3 p orbital is involved. So, the mode of hybridization is sp3.
Hybridization of P