Types Of Buffer Solution
There are two buffer forms, acid buffer, and base buffer.
Acid Buffer
A buffer solution that contains large quantities of a weak acid, and its salt with a strong base, is called an acid buffer. On the acidic side such buffer solutions have pH, i.e.pH is below 7 at 298 K. The equation gives the pH of an acid buffer. CH3COOH, with CH3COONa.
pH = pKa + ln\}\]
Where Ka —–acid dissociation constant of the weak acid
Basic Buffer
A buffer solution which contains relatively large quantities of a weak base and its salt with a strong acid is called a simple buffer. On the alkaline side these buffers have pH, i.e., pH is higher than 7 at 298 K. For example, NH4OH and NH4Cl
The pH of an appropriate buffer is determined by the equation
pOH = pKb + ln\}\]
Where, Kb ——base dissociation constant.These equations are called Henderson Hasselbalch equations
Ice Tables: A Useful Tool For Solving Equilibrium Problems
ICE tables are very helpful tools for understanding equilibrium and for calculating the pH of a buffer solution. They consist of using the initial concentrations of reactants and products, the change they undergo during the reaction, and their equilibrium concentrations. Consider, for example, the following problem:
Calculate the pH of a buffer solution that initially consists of 0.0500 M NH3 and 0.0350 M NH4+. . The equation for the reaction is as follows:
\text_4^+ \rightleftharpoons \text^+ + \text_3
We know that initially there is 0.0350 M NH4+ and 0.0500 M NH3. Before the reaction occurs, no H+ is present so it starts at 0.
ICE table initial: ICE table for the buffer solution of NH4+ and NH3 with the starting concentrations.
During the reaction, the NH4+ will dissociate into H+ and NH3. Because the reaction has a 1:1 stoichiometry, the amount that NH4+ loses is equal to the amounts that H+ and NH3 will gain. This change is represented by the letter x in the following table.
ICE table change: Describes the change in concentration that occurs during the reaction.
Therefore the equilibrium concentrations will look like this:
ICE table equilibrium: Describes the final concentration of the reactants and products at equilibrium.
Apply the equilibrium values to the expression for Ka.
} = \frac = \frac )} }
Assuming x is negligible compared to 0.0500 and 0.0350 the equation is reduced to:
} = \frac = \frac }
Solving for x :
x = = 3.92 x 10-10
pH = -log
pH = 9.41
What Is The Purpose Of A Buffer
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the pH of the solution relatively stable. This is important for processes and/or reactions which require specific and stable pH ranges.
Also Check: Does Mj Have Any Biological Kids
The Slow And Stupid Method
To avoid adding extra salt to a solution, prepare a buffer composed of an acid and its salt by dissolving the acid form of the buffer in about ~60% of the water required for the final solution volume. Adjust the pH using a strong base, such as NaOH. When preparing a buffer composed of a base and its salt, start with the base form and adjust the pH with strong acid, such as HCl. After the pH is correct, dilute to just under the final solution volume. Check the pH and correct if necessary, then add water to the final volume.
Advantages: Easy to understand.
Disadvantages: Slow. May require lots of base . If the base is concentrated, it is easy to overshoot the pH. If the base is dilute, it is easy to overshoot the volume. Ionic strength will be unknown. Adding a strong acid or base can result in temperature changes, which will make pH readings inaccurate unless the solution is brought back to its initial temperature.
Advantages: Fast. Easy to prepare. Additional pH adjustment is rarely necessary, and when necessary, the adjustment is small. Ionic strength easily calculated.
The Two Solution Method Make separate solutions of the acid form and base form of the buffer, both solutions having the same buffer concentration as the concentration of total buffer in the final solution. To obtain the desired pH, add one solution to the other while monitoring the pH with a pH meter.
Advantages: Easy to do.
Advantages: Easy to do . Convenient for frequently prepared buffers.
2.75
Role Of Buffer In Vitro:
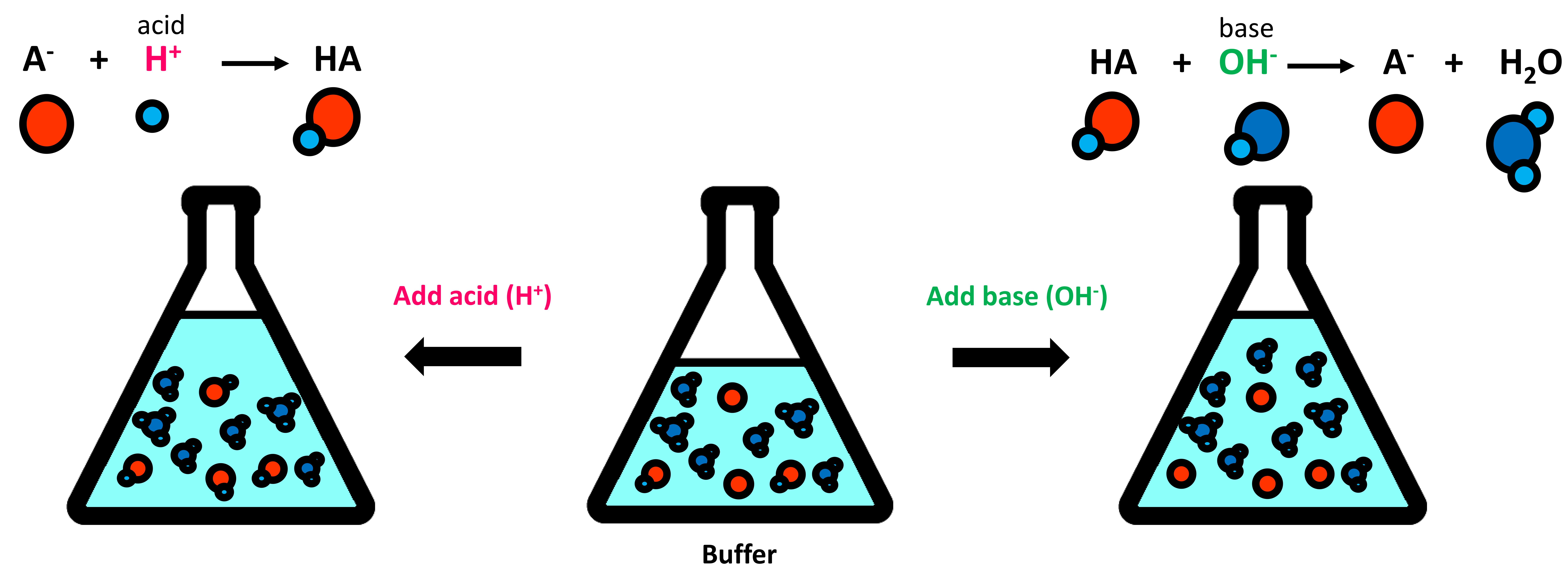
- Acid-base buffer helps in tissue or organ preservation
- In tissue culture, the optimum pH is maintained by buffers. For example HEPES buffer is widely used in cell culture because it is better at maintaining physiological pH despite change in CO2 concentration in media.
- HEPES also maintains enzyme structure and function at low temperature
- Buffer are used in fermentation process
- It is also used for setting correct condition for dyes used in coloring fabrics
- Buffer is used to calibrate pH meter
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
Control Of Ph By Buffer
Buffers are chemicals that, when added to water, tend to maintain a certain pH. This is due to the buffers ability to either accept or donate a proton or hydroxyl to keep the pH in a certain range. Buffers are different than strong acids and bases because buffers do not donate all of their protons or hydroxyls within their buffering range.
The Alkalinity Of A Solution
The alkalinity of a solution is dependent upon the number of hydroxyl ions present. Water is a neutral solution because each molecule contains one H+ and one OH. For each molecule of water dissociated, there is one H+ and one OH, each one neutralizing the other.
A neutral solution, such as water, where the number of hydrogen ions is balanced by the same number of hydroxyl ions, has a pH of 7.0. The range of the pH scale is from 0 to 14.
The pH scale runs from 1 to 14 neutrality being at pH 7.0.
Solutions having a pH lesser than 7 are acidic, i.e., the concentration of H+ is greater than that of OH.
Conversely, solutions having a pH more than 7 are basic or alkaline, i.e., denote an excess of OH over H+
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
What Is Buffer In Biology
An acid-base balancing or control reaction by which the pH of a solution is protected from major change when acid or base is added to it.
The protection is afforded by the presence in the solution of a weak acid and related salt , which maintains the equilibrium by means of ion transfer and neutralization.
The same effect can be obtained by the use of a blend of two acid salts phosphates, carbonates, and ammonium salts are common buffering agents.
the ability to prevent large changes in pH is an important property of most intact biological organisms.
The cytoplasmic fluid which contains dissolved proteins, organic substrates, and inorganic salts resist excessive changes in pH.
The blood plasma is a highly effective buffer solution almost ideally designed to keep the range of pH of the blood between 7.2 to 7.3.
In animals, a complex and vital buffer system is found in the circulating blood. The components of this system are CO2HCO3: Na2HPO4 the oxygenated and mono-oxygenated forms of hemoglobin, and the plasma proteins.
Many commercial products are approximately buffered to retain their original strength.
The Action Of Buffers In Blood Plasma
When carbon dioxide dissolves in the blood, it decreases the pH value, thereby increasing the acidic content of the blood.
In this case, alkaline buffers come into play. They tend to mix with the plasma of blood and then neutralize its value.
The same happens in the plasma when the alkaline value of blood increases. In this case, acidic buffers in the blood plasma play their role.
If the alkalinity or the acidity of blood pertains for a longer period, the body gets into a hazardous state, which if left unaddressed, can prove fatal.
Read Also: Hawkes Learning System Software
Preparing A Buffer Solution
There are a couple of ways to prepare a buffer solution of a specific pH. In the first method, prepare a solution with an acid and its conjugate base by dissolving the acid form of the buffer in about 60% of the volume of water required to obtain the final solution volume. Then, measure the pH of the solution using a pH probe. The pH can be adjusted up to the desired value using a strong base like NaOH. If the buffer is made with a base and its conjugate acid, the pH can be adjusted using a strong acid like HCl. Once the pH is correct, dilute the solution to the final desired volume.
pH probe
Alternatively, you can prepare solutions of both the acid form and base form of the solution. Both solutions must contain the same buffer concentration as the concentration of the buffer in the final solution. To get the final buffer, add one solution to the other while monitoring the pH.
In a third method, you can determine the exact amount of acid and conjugate base needed to make a buffer of a certain pH, using the Henderson-Hasselbach equation:
pH=p_+log
where pH is the concentration of , pKa is the acid dissociation constant, and and are concentrations of the conjugate base and starting acid.
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
Boundless.
Introduction: What Is A Buffer Solution
A buffer is an aqueous solution that consists of a mixture of a weak acid and its salt or a weak base with its salt . Its pH changes very little when a small amount of strong acid or base is added to it and is thus used to prevent a solution ‘s pH change.
Buffer solutions are used for a wide range of chemical applications. Blood is one example of a buffer solution found in nature. Human blood has natural pH of 7.4. Many people experience severe anxiety and suffer from alkalosis. Alkalosis is a disease in which blood pH is excessively high. The reverse condition is called acidosis-a blood,pH greater than 7.4
Some chemical reactions only occur at a certain pH. Other households and consumer items need to monitor their pH values, such as shampoo to combat the soap’s alkalinity to avoid inflammation, baby lotion to retain a pH of around 6 to discourage multiplication of bacteria, washing powder, eye drops, fizzy lemonade etc.
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
Preparation Of Acid Buffer
Consider an acid buffer solution, containing a weak acid and its salt with a strong base. Weak acid HA ionizes, and the equilibrium can be written as-
HA + H2O H+ + A
Acid dissociation constant = Ka = /HA
Taking, negative log of RHS and LHS:
pH of acid buffer = pKa +
The equation is the Henderson-Hasselbalch equation, popularly known as the Henderson equation.
What Are Examples Of Biological Buffers
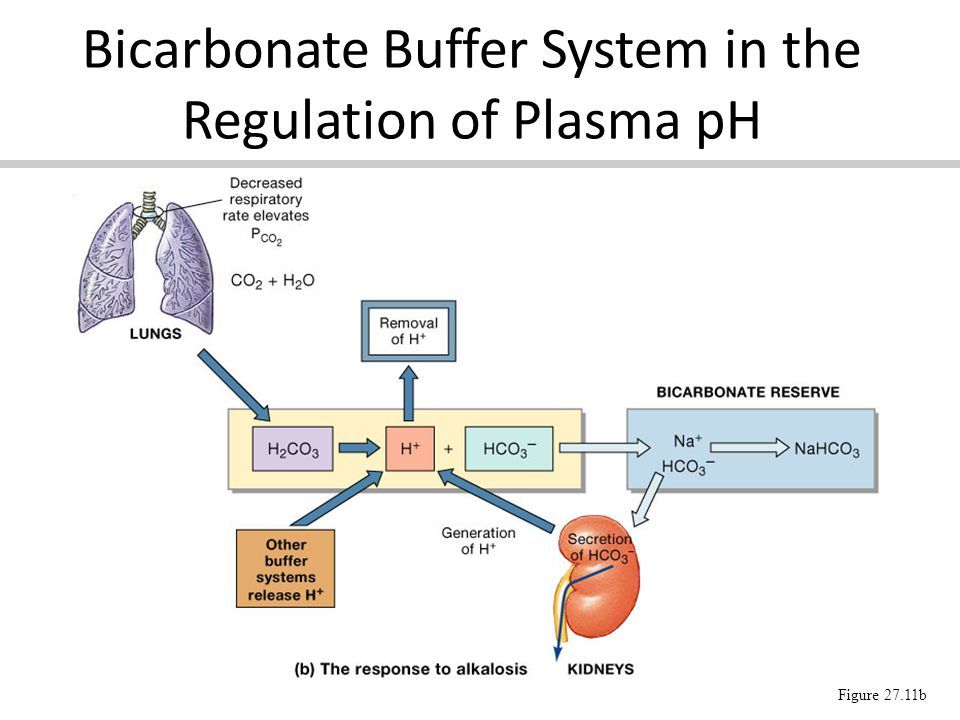
There are many different biological buffers manufactured and sold for the express purpose of maintaining physiological pH. This is very important for a scientist working with biologically relevant materials. These buffers do well to maintain a steady pH near 7.4. A very commonly used biological buffer is called HEPES -1-piperazineethanesulfonic acid). This buffer is very good at maintaining a steady pH between 6.8 and 8.2.
Buffers should be chosen based on knowledge of the pH that you want to work at and the range over which you need to have a steady pH.
Related Articles
You May Like: What Is The Molecular Geometry Of Ccl4
There Are Two Types Of Buffer Solutions
Acidic buffer
Acid buffer solutions have a pH less than 7. It is generally made from a weak acid and one of its salts . Commonly used acidic buffer solutions are a mixture of ethanoic acid and sodium ethanoate in solution, which have a pH of 4.76 when mixed in equal molar concentrations. You can change the pH of the buffer solution by changing the ratio of acid to salt, or by choosing a different acid and one of its salts.
Alkaline buffer
Alkaline buffer solutions have a pH greater than 7 and are made from a weak base and one of its salts. A very commonly used example of an alkaline buffer solution is a mixture of ammonia and ammonium chloride solution. If these were mixed in equal molar proportions, the solution would have a pH of 9.25.
What Are Biological Buffers
Remember the story of Goldilocks and the three bears? When Goldilocks goes into the bears’ house, she tries the porridge and finds that the first is too hot, and the second is too cold. When she tries the third one, she finds a porridge that is just right. In the same way Goldilocks wanted porridge that was perfect to eat, organisms want an environment that is just right. But what is just right for an organism?
Recommended Reading: Geometry Dash Password Hack
Mechanism Of Buffering Action
Consider the example of a buffer solution made by dissolving sodium acetate into acetic acid, to consider how a buffer functions. As you can see from the name, acetate acid is an acid: CH3COOH, while sodium acetate dissociates in solution to yield the conjugate base, CH3COO-acetate ions. The reaction equation is:
CH3COOH + OH- CH3COO- + H2O
If this solution is combined with a strong acid, the acetate ion can neutralize:
CH3COO- + H+ CH3COOH
It changes the original buffer reaction equilibrium, thereby holding the pH steady.
Biological Buffers And Ph Level
Biological buffers are organic substances that maintain a constant pH over a given range by neutralizing the effects of hydrogen ions. Buffers also provide a pH environment conducive to critical biochemical processes, wherein a significant change in pH can lead to a harmful change in molecular structure, biological activity or functions. They keep the pH constant by taking up protons which are released during reactions, or by releasing protons when they are consumed by reactions. Buffers are a vital component for modeling biological systems and have many uses in cell culture, molecular biology, nucleic acid, protein purification, transformation and transfections.
The quality of a buffer is determined by is resistance to changes in pH when strong acids or bases are added and is measured in the value of its pKa where the larger the value of pKa, the weaker the acid. Knowing the value of pKa is important in dealing with systems involving acid-base equilibria in solution. Most of the buffers used today were developed in 1966 by Norman Good and his colleagues who identified parameters and characteristics of effective buffers. These biological buffers are called Zwitterionic bufers.
Recommended Reading: What Is Elastic Force In Physics
Problems On Buffer Solution
Problem 1: What is the ratio of base to acid when pH = pKa in buffer solution? How about when pH = PKa + 1?
Sol:
pH = pKa when the ratio of base to acid is 1 because log 1 = 0.
When log = 1, then the ratio of base to acid is 10:1.
What is the pH of a buffered solution of 0.5 M ammonia and 0.5 M ammonium chloride when, enough hydrochloric acid corresponding to make 0.15 M HCl? The pKb of ammonia is 4.75.
pKa = 14 pKb. = 9.25
0.15 M H+ reacts with 0.15 M ammonia to form 0.15 M more ammonium.
So, ammonium ion is 0.65 M and 0.35 M remaining ammonia .
Using Henderson-Hasselbalch equation,
pKa log = 9.25 log = 9.25 .269 = 8.98
Problem 2: How many moles of sodium acetate and acetic acid must you use to prepare 1.00 L of a 0.100 mol/L buffer with pH 5.00.
Sol:
pH = pKa + log
5.00 = 4.74 + log
log = 0.26
Why Do Buffers Matter
Cells function in a narrow range of pH. So do the many enzymes that help you digest food, make energy and relay signals between nerve cells. As such, it is vital that the pH in the body and in these cells does not vary drastically. Otherwise, they’ll stop doing the work you need them to do. To make sure that this does not happen, buffers are found in all biologically relevant solutions.
Biological buffers can also be buffer systems that help maintain a steady pH around the physiological pH. When conducting experiments with individual components of cells or individual proteins, scientists must take into account the buffer they use. Without a good buffer, the activity of the component they want to study may decrease.
For example, if a scientist is working with a protein that functions in the brain at a pH of 7.4, but the scientist uses a buffer of pH 8.0, then the protein will not function optimally. Instead the scientist should switch to a buffer closer to the pH that the protein actually functions in. This way the scientist can observe the protein functioning in an environment similar to its natural environment.
Read Also: Geometry Segment Addition Postulate Worksheet
What Does Ph Mean In A Buffer
In chemistry, pH is a measure of the hydrogen ion concentration in a solution. The pH of a buffer can be calculated from the concentrations of the various components of the reaction. The balanced equation for a buffer is:
\text \rightleftharpoons \text^+ + \text^-
The strength of a weak acid is usually represented as an equilibrium constant. The acid-dissociation equilibrium constant , which measures the propensity of an acid to dissociate, for the reaction is:
\text_} = \frac
The greater x is than , the greater the value of Ka, the more the formation of H+ is favored, and the lower the pH of the solution.
What Is The Function Of A Buffer In Biology
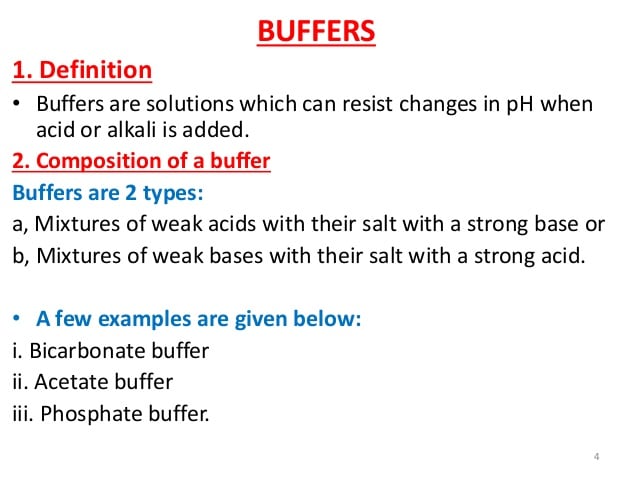
A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological buffer helps maintain the body at the correct pH so that biochemical processes continue to run optimally. They help maintain a given pH even after the addition of an acid or a base.
Don’t Miss: What Does Abiotic Mean In Biology