S To Measure And Detect
For Transcription, RT-PCR, DNA microarray, In-situ hybridization, Northern blot, RNA-Seq is quite often used for measurement and detection.For Translation, western blotting, immunoblotting, enzyme assay, Protein sequencing, Metabolic labeling, proteomics is used for measurement and detection.
Cricks central dogma: DNA —> Transcription —> RNA —> Translation —> Protein
Genetic code used during translation:
Where In The Cell Does Transcription And Translation Take Place
Replication and transcription take place in the nucleus while translation takes place in the cytoplasm for eukaryotic cells.
Where is the tRNA located in the cell?
tRNA or Transfer RNA. Like rRNA, tRNA is located in the cellular cytoplasm and is involved in protein synthesis. Transfer RNA brings or transfers amino acids to the ribosome that corresponds to each three-nucleotide codon of rRNA.
Amino Acids Are Added To The C
Having seen that amino acids are first coupled to molecules, we now turn to the mechanism by which they are joined together to form proteins. The fundamental of synthesis is the formation of a between the at the end of a growing chain and a free on an incoming . Consequently, a protein is synthesized stepwise from its N-terminal end to its C-terminal end. Throughout the entire process the growing carboxyl end of the polypeptide chain remains activated by its covalent attachment to a tRNA . This high-energy covalent is disrupted during each addition but is immediately replaced by the identical linkage on the most recently added amino acid . In this way, each amino acid added carries with it the for the addition of the next amino acid rather than the energy for its own additionan example of the head growth type of polymerization described in .
The incorporation of an amino acid into a protein. A polypeptide chain grows by the stepwise addition of amino acids to its C-terminal end. The formation of each peptide bond is energetically favorable because the growing C-terminus has been activated
You May Like: Why Geography Matters More Than Ever Chapter 1
Trnas: The Interpreter Of The Code
While the ribosomes are the factories that join amino acids together using the instructions in mRNAs, another class of RNA molecules, the transfer RNAs are also needed for translation.
In terms of the bead analogy above, someone or something has to be able to bring a red bead in when the instructions indicate UGG, and a green bead when the instructions say UUU. This, then, is the function of the tRNAs.
They act as ADAPTORS or interpreters of the code. They act as adaptors by binding to the codon on one end and carrying the amino acid on the other end.
There is at least one tRNA for each amino acid.
All transfer RNAs share common features and are structurally similar. These include
1. Transfer RNAs are small single-stranded RNA molecules, about 75-90 nucleotides long.
2. Transfer RNAs are extensively modified post-transcriptionally and contain a large number of unusual bases and modified bases like Inosine.
3. Mature tRNAs take on a three-dimensional structure where the single-stranded tRNA folds on itself and base-pairs to form what is sometimes described as a stem-loop, or cloverleaf structure.
This structure is crucial to the function of the tRNA, providing both the sites for attachment of the appropriate amino acid and for recognition of codons in the mRNA.
The cloverleaf consists of five parts: the acceptor stem , the D-arm, the anticodon arm, the variable loop and the TC-arm .
5. For all tRNA amino acid is attached to a hydroxyl group of the A .
Figure 10. 5
Where In The Cell Does Translation Take Place Quizlet
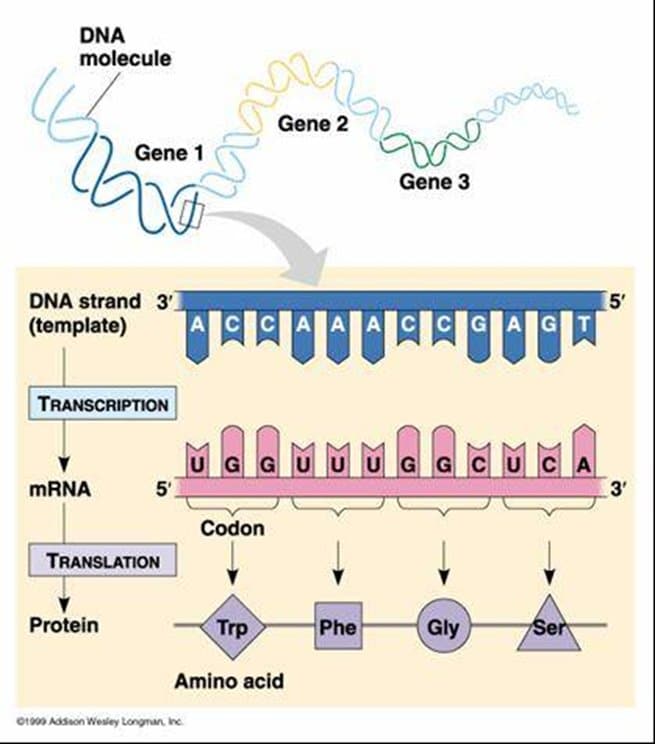
In a eukaryotic cell, translation occurs in the ribosomes that are in the cell cytoplasm and the endoplasmic reticulum. Translation is the process where the codons of the mRNA are decoded. The codons are translated to the language of amino acids from the language of nucleic acids.
Where is translation in biology?
the ribosomeIn prokaryotes , translation occurs in the cytosol, where the large and small subunits of the ribosome bind to the mRNA. In eukaryotes, translation occurs in the cytoplasm or across the membrane of the endoplasmic reticulum in a process called co-translational translocation.
What is translation and where does it take place quizlet?
converts mRNA into a protein. Translation takes place in the . can leave the nucleus. converts DNA into mRNA.
What is translation and where does it occur in the cell?
In molecular biology and genetics, translation is the process in which ribosomes in the cytoplasm or ER synthesize proteins after the process of transcription of DNA to RNA in the cells nucleus.
Don’t Miss: What Is Taxonomy In Biology Class 11
Nucleotide Sequences In Mrna Signal Where To Start Protein Synthesis
The initiation and termination of translation occur through variations on the translation elongation cycle described above. The site at which synthesis begins on the is especially crucial, since it sets the for the whole length of the message. An error of one either way at this stage would cause every subsequent in the message to be misread, so that a nonfunctional protein with a garbled sequence of amino acids would result. The initiation step is also of great importance in another respect, since for most genes it is the last point at which the cell can decide whether the mRNA is to be translated and the protein synthesized the rate of initiation thus determines the rate at which the protein is synthesized. We shall see in Chapter 7 that cells use several mechanisms to regulate translation initiation.
The translation of an begins with the AUG, and a special is required to initiate translation. This always carries the methionine so that all newly made proteins have methionine as the first amino acid at their N-terminal end, the end of a that is synthesized first. This methionine is usually removed later by a specific protease. The has a sequence distinct from that of the tRNA that normally carries methionine.
The initiation phase of protein synthesis in eucaryotes. Only three of the many translation initiation factors required for this process are shown. Efficient translation initiation also requires the poly-A tail of the mRNA bound by poly-A-binding proteins
Rrna Trna And Mrna Differences
As previously mentioned rRNA, tRNA, and mRNA are all RNA molecules, but each has different functions. rRNAs make up ribosomes. Ribosomes have a large subunit known as 50S and a small subunit known as 30S. Each ribosomal subunit is comprised of its own rRNA sequence. Within the ribosome, there are two different types of rRNAs: small rRNAs and large rRNAs. These rRNAs make up the small and large subunits respectively. These specialized tRNAs transfer amino acids during the translation of mRNA into proteins. Each of the 20 amino acids binds to a specific tRNA and is deposited onto the growing peptide chain during translation. Lastly, mRNA houses the blueprint of your genes that will be translated into functional proteins needed for cell signaling and overall metabolism.
Read Also: What Is Integrated Math 3 Equivalent To
Explain: Dna To Mrna To Protein
Transcription is a process that converts DNA information into a messenger RNA molecule in the initial stage. During transcription, RNA polymerase II catalyses the synthesis of a pre-mRNA molecule, later processed to become mature mRNA. The DNA of a gene acts as a template for complementary base pairing. The resulting mRNA contains a single-stranded copy of the gene that needs to be converted into a protein molecule. The mRNA is read following the genetic code, which connects the DNA sequence to the amino acid sequence in proteins during translation, the second major stage in gene expression. A codon is a collection of three nucleotides found in mRNA, and each codon encodes an individual amino acid .
What Is A Translation In Science
Listen to pronunciation. In biology, the process by which a cell makes proteins using the genetic information carried in messenger RNA . The mRNA is made by copying DNA, and the information it carries tells the cell how to link amino acids together to form proteins.
What is the purpose of a translation?
The purpose of translation is to convey the original intent of a message, taking into account cultural and regional differences between languages. Translation has been used by humans for millennia, beginning with the appearance of written language.
What is translation and its steps?
Translation is the process by which the genetic code contained within a messenger RNA molecule is decoded to produce a specific sequence of amino acids in a polypeptide chain. It occurs in the cytoplasm following DNA transcription and, like transcription, has three stages: initiation, elongation and termination.
What happens first in translation?
mRNA synthesized in the nucleus as per the sequence of codons in DNA. It moves to ribosoms in cytoplasm for the process of translation. Translation is the synthesis of protein with specific sequence of amino acids. The sequence of amino acids is determined by the sequence of nucleotids in DNA, that constitute gene.
What is translation in science definition?
What is the outcome of translation?
Read Also: What Happened To Beth Thomas Biological Father
Specific Enzymes Couple Each Amino Acid To Its Appropriate Trna Molecule
We have seen that, to read the in , cells make a series of different tRNAs. We now consider how each becomes linked to the one in 20 that is its appropriate partner. Recognition and attachment of the correct amino acid depends on enzymes called , which covalently couple each amino acid to its appropriate set of tRNA molecules . For most cells there is a different synthetase for each amino acid one attaches glycine to all tRNAs that recognize codons for glycine, another attaches alanine to all tRNAs that recognize codons for alanine, and so on. Many bacteria, however, have fewer than 20 synthetases, and the same synthetase enzyme is responsible for coupling more than one amino acid to the appropriate tRNAs. In these cases, a single synthetase places the identical amino acid on two different types of tRNAs, only one of which has an that matches the amino acid. A second enzyme then chemically modifies each incorrectly attached amino acid so that it now corresponds to the anticodon displayed by its covalently linked tRNA.
The structure of the aminoacyl-tRNA linkage. The carboxyl end of the amino acid forms an ester bond to ribose. Because the hydrolysis of this ester bond is associated with a large favorable change in free energy, an amino acid held in this way is said
Molecular Chaperones Help Guide The Folding Of Many Proteins
The folding of many proteins is made more efficient by a special class of proteins called molecular chaperones. The latter proteins are useful for cells because there are a variety of different paths that can be taken to convert the molten globule form of a to the protein’s final compact . For many proteins, some of the intermediates formed along the way would aggregate and be left as off-pathway dead ends without the intervention of a chaperone that resets the folding process .
A current view of protein folding. Each domain of a newly synthesized protein rapidly attains a molten globule state. Subsequent folding occurs more slowly and by multiple pathways, often involving the help of a molecular chaperone.
Molecular chaperones were first identified in bacteria when E. coli mutants that failed to allow bacteriophage lambda to replicate in them were studied. These cells produce slightly altered versions of the chaperone machinery, and as a result they are defective in specific steps in the assembly of the viral proteins. The molecular chaperones are included among the heat-shock proteins , because they are synthesized in dramatically increased amounts after a brief exposure of cells to an elevated temperature . This reflects the operation of a feedback system that responds to any increase in misfolded proteins by boosting the synthesis of the chaperones that help these proteins refold.
Recommended Reading: What Is C5 In Chemistry
Stages Of The Translation Process
The process Translation utilizes the components of Translation during the following three stages.
Termination
Initiation: For initiation, the Ribosome binds to the mRNA. This binding happens at the start codon – a stretch of three nucleobases namely Adenine, Uracil and Guanine . This starting code is only recognized by the initiator tRNA which carries the methionine amino acid with it. Hence, usually in most cases, the sequence of a Protein starts with methionine.
Elongation: This stage is characterized by the addition of amino acids one by one and the formation of a polypeptide chain in a sequence determined by the DNA and represented by the RNA. In this stage, complexes made up of amino acids bound to tRNA sequentially attach to the next in line three pairs of nucleobase or called as codons on the mRNA and the Ribosome moves from codon to codon on the mRNA producing the polypeptide.
Termination: In this last stage, a release factor binds to the stop codon, and termination of Translation and releasing the complete polypeptide from the Ribosome.
Third Step In Translation : Termination
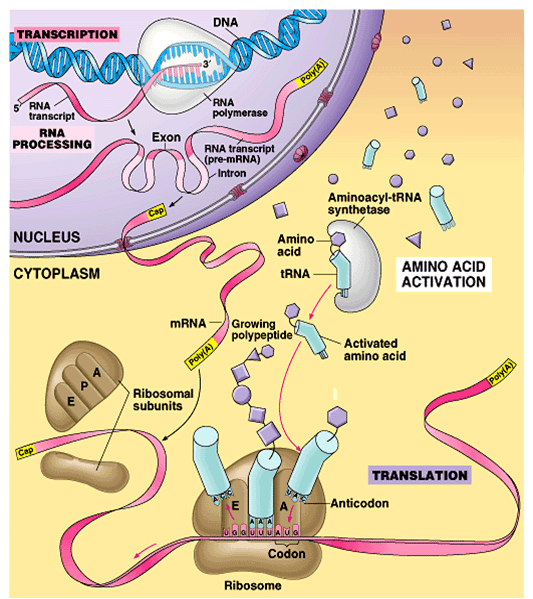
Translation ends during the termination stage. Termination takes place when a nonsense or stop codon enters the A site. Release factors recognize these nonsense codons and signal the hydrolysis of the bond between the tRNA and the P site polypeptide chain. This releases the newly made protein, which needs to be folded to function.
Protein folding is the process by which the polypeptide chain is folded into its specific three-dimensional structure. This takes place during and after translation.
The small and large ribosomal subunits then detach from each other and from the mRNA before taking part in another translation initiation complex. When ribosomes complete translation, the mRNA becomes degraded so its nucleotides can participate in another transcription reaction.
Don’t Miss: What Is Conjugation In Biology
What Happens To Mrna After Translation
Messenger RNA mediates the transfer of genetic information from the cell nucleus to ribosomes in the cytoplasm, where it serves as a template for protein synthesis. Once mRNAs enter the cytoplasm, they are translated, stored for later translation, or degraded. All mRNAs are ultimately degraded at a defined rate.
Why Is Translation Important In Biology
The process of translation can be seen as the decoding of instructions for making proteins, involving mRNA in transcription as well as tRNA. The genes in DNA encode protein molecules, which are the workhorses of the cell, carrying out all the functions necessary for life.
Read Also: Who Is Generally Recognized As The Founder Of American Psychology
First Step In Translation : Initiation
Translation starts with ribosomal assembly, leading to the formation of the initiation complex. The formation of the initiation complex is as follows:
The small ribosomal subunit binds to the initiator tRNA molecule , which contains the amino acid methionine in archaea and eukaryotes and the N-formyl-methionine in bacteria. The tRNAi is charged because it carries an amino acid.
The small ribosomal subunit bound to the charged tRNAi travels across the mRNA strand up to the start codon AUG, which signifies the beginning of translation. AUG also marks the beginning of the reading frame .
The anticodon on the tRNAi binds to the start codon through base pairing. The anticodon is a codon in the tRNA that is complementary to a codon in the mRNA.
The small ribosomal subunit, mRNA, and charged tRNAi bind to the large ribosomal subunit. This forms the initiation complex. Proteins calledinitiation factors bring together these components. The cell uses energy in the form of Guanosine triphosphate to fuel the process.
What Is Reverse Transcription
Reverse transcription is the process of transcribing a DNA molecule from an RNA molecule. This method of replication is utilized by retroviruses, such as HIV, and produces altered DNA, which can be incorporated directly into a host cell, allowing rapid reproduction. This is made possible by the reverse transcriptase enzyme. This can be seen in Figure 4.
Figure 4: The process of reverse transcription.
Recommended Reading: Algebra 2 Chapter 7 Test Answer Key
What Are The 6 Steps Of Translation
Translation is executed in six steps: binding of mRNA to ribosome, aminoacylation, initiation, elongation, termination and post-translational modification, Binding of mRNA to ribosome. I. binding of mRNA to ribosome.
What is made during the process of translation?
Translation. The molecule that results from translation is protein or more precisely, translation produces short sequences of amino acids called peptides that get stitched together and become proteins. During translation, little protein factories called ribosomes read the messenger RNA sequences. Every three RNA bases code for an amino acid.
What are the steps of the initiation of translation?
The first step in translation, initiation, begins when an mRNA binds to a free light ribosomal subunit. A transfer RNA molecule then brings the first amino acid to the light subunit of the ribosome. Other protein factors join this assemblage and then the heavy ribosomal subunit binds to complete initiation.
The Proteasome Degrades A Substantial Fraction Of The Newly Synthesized Proteins In Cells
Cells quickly remove the failures of their translation processes. Recent experiments suggest that as many as one-third of the newly made chains are selected for rapid degradation as a result of the quality control mechanisms just described. The final disposal apparatus in eucaryotes is the , an abundant ATP-dependent protease that constitutes nearly 1% of cellular protein. Present in many copies dispersed throughout the and the , the also targets proteins of the : those proteins that fail either to fold or to be assembled properly after they enter the ER are detected by an ER-based surveillance system that retrotranslocate them back to the cytosol for degradation .
The proteasome. A cut-away view of the structure of the central 20S cylinder, as determined by x-ray crystallography, with the active sites of the proteases indicated by red dots. The structure of the entire proteasome, in which the central cylinder
The 19S caps act as regulated gates at the entrances to the inner proteolytic chamber, being also responsible for binding a targeted to the . With a few exceptions, the proteasomes act on proteins that have been specifically marked for destruction by the covalent attachment of multiple copies of a small protein called . Ubiquitin exists in cells either free or covalently linked to a huge variety of intracellular proteins. For most of these proteins, this tagging by ubiquitin results in their destruction by the proteasome.
Also Check: What Was The First School Of Thought In Psychology