Why Does P Mean Log
$\mathrm = -\log$ while $\mathrm = -\log$
Why does $\mathrm p$ represent $-\log$ of something? Is it due to some historical reason or due to some scientific reason?
- 1$\begingroup$@TanMath Today, the symbol $\mathrm p$ is interpreted as an operator . If you are interested in the etymology of this use, you might get better answers on hsm.stackexchange.com.$\endgroup$ user7951Sep 25, 2015 at 19:48
- $\begingroup$@Loong can you give me evidence for your claim? I cant find anything about p being an operator $\endgroup$Sep 25, 2015 at 19:51
- 1$\begingroup$@TanMath You can find this definition in the international standard ISO 80000-9 Quantities and units Part 9: Physical chemistry and molecular physics as well as in the IUPAC Green Book.$\endgroup$ user7951Sep 25, 2015 at 19:57
- $\begingroup$@Loong is that online?$\endgroup$Sep 25, 2015 at 19:59
- 1$\begingroup$@TanMath The Green Book is available online: iupac.org/fileadmin/user_upload/publications/e-resources/$\endgroup$ user7951
According to the international standard ISO 80000 Quantities and units Part 9: Physical chemistry and molecular physics, the symbol $\mathrm p$ is interpreted as an operator $$. It is used in particular in the definition of the quantity $\mathrm$:
$$\mathrm=\mathrm pa_}=-\lg\left=-\lg\left.$$
The definition of $\mathrm p$ given by ISO is actually quoted from IUPAC Quantities, Units and Symbols in Physical Chemistry .
Kw Of Water And Temperature Changes
One thing you need to appreciate when looking at Kw definition chemistry and Kw constant is that the PH varies depending on temperatures. Using the equation , the first equation in this post, a forward reaction absorbs heat.
H2O+H2OH3O++OH
Using Le Chateliers Principle, you will find that raising the temperature of water results in the equilibrium working to lower the temperature. This is achieved through heat absorption. It also implies that forward reaction is going to be favored The overall impact will be lowering of Kw as the temperature moves up. To demonstrate the changes to Kw as temperature changes, have a look at the table below:
| T |
Isotopes Of The Hydrogen Ion
- The most common isotope of hydrogen in nature contains a proton without any neutrons in the nucleus: 1H is called protium, distinguished from the second stable isotope deuterium 2H, and the third naturally occuring isotope is tritium 3H with a half-life of 12.32 years. The dissociation constant of heavy water differs from normal water . 1H+ and 2H+ are hydrogen ions H+, but 2H+ is more than a proton. Respiratory complex IV and the other pumps in the electron transfer system pump not only protons, but pump 2H+
Also Check: Chapter 6 Mid Chapter Test Glencoe Algebra 2
Acid Dissociation Constant From Ph
The pH scale, or power of hydrogen, is a numerical measure of a solutions acidity or basicity. In an aqueous solution, it can be used to compute the concentration of hydrogen ions or hydronium ions . Low pH solutions are the most acidic, whereas high pH solutions are the most basic.
The concentration of free hydrogen ions in an aqueous acid solution is measured by its pH: pH equals -log or -log . The last equation can be rewritten as follows:
The above comparison allows you to determine the relative concentration of acid to conjugate base and estimate the dissociation constant Ka if you know the molar concentration of an acid solution and can detect its pH.
Using Ka And Pka To Predict Equilibrium And Strength Of Acids
Ka may be used to measure the position of equilibrium:
- If Ka is large, the formation of the products of the dissociation is favored.
- If Ka is small, the undissolved acid is favored.
Ka may be used to predict the strength of an acid:
- If Ka is large this means the acid is mostly dissociated, so the acid is strong. Acids with a pKa less than around -2 are strong acids.
- If Ka is small , little dissociation has occurred, so the acid is weak. Acids with a pKa in the range of -2 to 12 in water are weak acids.
Ka is a better measure of the strength of an acid than pH because adding water to an acid solution doesn’t change its acid equilibrium constant, but does alter the H+ ion concentration and pH.
Don’t Miss: Where Does Psychology Focus Its Attention
The Concentration Of Hydrogen Ion
The concentration of Hydrogen Ion lets us determine the concentration of acidity or alkaline structure of the ion. Let us understand this with the help of an example. The most common and relatable example would be water . Most of the water molecules are in a stable and known shape. Its deeper compounds are broken into hydrogen ions and hydroxide ions, that is, H + and OH-.
In fact, the pH of water is determined by the balance of hydrogen and hydroxide ions.
The solution is acidic when the hydrogen ions outnumber the hydroxide ions. If the situation is reversed, the solution is alkaline. For any solution, the following relationship between the densities of hydrogen ions and hydroxide ions is observed if the temperature does not change: =Kw=10-14 at 25oC
In pure water or neutral solution-
===10-14=10-7
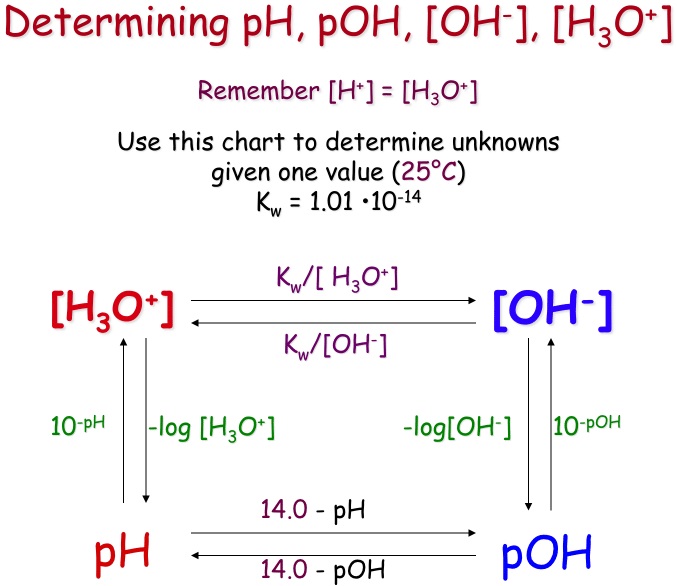
If the value of either or is known, the value of the other can be determined.
Thus pH is determined by hydrogen ion concentration
pH=-log10
What Does The Brackets Around And Represent And What Does It Do
In this context, the brackets means #”concentration”#
It is tempting to assume that #HO^-# and #H_3O^+# are actual species in aqueous species. As far as anyone knows these species are CLUSTERS of water molecules LESS or PLUS a proton…
And so or …or something…we use the #HO^”/”H_3O^+# as a label of convenience…the acidium species is a water cluster associated with an extra proton, and the hydroxide species is a water cluster LESS a proton. And if you play rugby think of #H^+# as the ball in a maul.
But certainly, when we do acid-base titrations, we can find the equivalence of acids and bases, straightforwardly and quantitatively, using very simple equipment, calibrated burettes, and standard indicatos.
And we use the brackets to designate a concentration term: #=10^# under standard conditions. And for simplicity, when we see ##
Read Also: What Does Relativity Mean In Physics
Ph Value Or Hydrogen Ion
The pH value, or hydrogen ion concentration, determines the acidity of a water. It is one of the most important determinations in water chemistry as many of the processes involved in water treatment are pH dependent. Pure water is very slightly ionized into positive hydrogen ions and negative hydroxyl ions. In very general terms a solution is said to be neutral when the numbers of hydrogen ions and hydroxyl ions are equal, each corresponding to an approximate concentration of 107 moles/l. This neutral point is temperature dependent and occurs at pH 7.0 at 25°C. When the concentration of hydrogen ions exceeds that of the hydroxyl ions the water has acidic characteristics. Conversely, when there is an excess of hydroxyl ions the water has basic characteristics and is described as being on the alkaline side of neutrality.
The pH value of unpolluted water is mainly determined by the inter-relationship between free carbon dioxide and the amounts of carbonate and bicarbonate present . The pH values of most natural waters are in the range 49, with soft acidic waters from moorland areas generally having lower pH values and hard waters which have percolated through chalk or limestone generally having higher pH values.
P.D. Davis BSc CPhys MIstP MIPSM, … G.N.C. Kenny BSc MD FRCA, in, 1995
Limitations To The Arrhenius Theory
The Arrhenius theory has many more limitations than the other two theories. The theory suggests that in order for a substance to release either H+ or OH- ions, it must contain that particular ion. However, this does not explain the weak base ammonia which, in the presence of water, releases hydroxide ions into solution, but does not contain OH- itself.
Hydrochloric acid is neutralized by both sodium hydroxide solution and ammonia solution. In both cases, you get a colourless solution which you can crystallize to get a white salt – either sodium chloride or ammonium chloride. These are clearly very similar reactions. The full equations are:
In the sodium hydroxide case, hydrogen ions from the acid are reacting with hydroxide ions from the sodium hydroxide – in line with the Arrhenius theory. However, in the ammonia case, there are no hydroxide ions!
You can get around this by saying that, when the ammonia reacts with the water, it is dissolved in to produce ammonium ions and hydroxide ions:
This is a reversible reaction, and in a typical dilute ammonia solution, about 99% of the ammonia remains as ammonia molecules. Nevertheless, there are hydroxide ions there, and we can squeeze this into the Arrhenius theory. However, this same reaction also happens between ammonia gas and hydrogen chloride gas.
Recommended Reading: What Is Difference Threshold In Psychology
How Ph Is Measured
Rough pH measurements can be made using litmus paper or another type of pH paper known to change colors around a certain pH value. Most indicators and pH papers are useful only to tell whether a substance is an acid or a base or to identify pH within a narrow range. A universal indicator is a mixture of indicator solutions intended to provide a color change over a pH range of 2 to 10.
More accurate measurements are made using primary standards to calibrate a glass electrode and pH meter. The electrode works by measuring the potential difference between a hydrogen electrode and a standard electrode. An example of a standard electrode is silver chloride.
What Is The Difference Between H And H+ In Chemistry
The hydrogen atom consists of a proton, which forms the nucleus, and an electron which is found outside the nucleus. A hydrogen ion, H+, is just the proton, with a positive charge. The hydrogen atom consists of a proton, which forms the nucleus, and an electron which is found outside the nucleus.Click to see full answer. Keeping this in consideration, what does H+ mean in chemistry?Acids. Acids add Hydrogen Ions to solutions. Hydrochloric acid splits into Hydrogen Ions and Chloride Ions . Extra H+ means acid solution .Also, what is the difference between H and h2? H2 is molecular hydrogen, which is mostly gaseous and extremely flammable. It is a molecule consisting two hydrogen atoms. Whereas H is hydrogen, neutral and an atom. Besides, which is more stable H+ or H? Originally Answered: Why is H+ ion more stable as compared to H- ion? Lets first think about it this way. A H+ ion will contain no electrons while a H- electron will contain more than 1 electrons. This is because the proton in the hydrogen atom can only stabilize 1 electron.How many electrons does H+ have?There are no electrons in a hydrogen ion. Hydrogen atoms are composed of one proton and one electron.
Read Also: Is Elton John The Biological Father
Iupac Definition Of Ph
The International Union of Pure and Applied Chemistry has a slightly different pH scale that is based on electrochemical measurements of a standard buffer solution. Essentially, the definition uses the equation:
pH = -log aH+
where aH+ stands for hydrogen activity, which is the effective concentration of hydrogen ions in a solution. This might be slightly different from the true concentration. The IUPAC pH scale also includes thermodynamic factors, which may influence pH.
For most situations, the standard pH definition is sufficient.
Related Content In Oxford Reference
Reference entries
PRINTED FROM OXFORD REFERENCE . Copyright Oxford University Press, 2021. All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use .
date: 30 November 2022
Recommended Reading: What Is The Formula For Velocity In Physics
What Is An Hydrogen Ion
A hydrogen ion is the nucleus of a hydrogen atom that has been isolated from its electron. A proton is a molecule with a unit of positive electric energy that makes the hydrogen nucleus. Therefore, the disconnected hydrogen ion, signified by the image H+, is usually used to depict a proton. So the above-given definition assists with getting what is H+ ion and hydrogen ion Formula.
When an isolated hydrogen atom exists, it is shown as an H+ ion. It can only function in spaces that are nearly particle-free, also known as high vacuum places or the gaseous state. This is because the mere nucleus can easily combine with other given particles or elements such as electrons, atoms, and molecules.
What Does Kw Mean In Chemistry
Water contains both acidic and basic molecules. Because acids and bases will always react when put together, it simply means that water will react with itself! This sounds very strange. But in reality, it actually happens. The water molecules exchange protons in a process referred to as autoionization of water. This process can be expressed in the following equation below:
H2O+H2OH3O++OH
In the above equation, one water molecule can be seen donating a proton and, therefore, acts as a Bronsted-Lowry acid. Then, another molecule accepts the molecule and, therefore, acts as a Bronsted-Lowry base. After the reaction, two molecules are formed, Hydronium ions and hydroxide ions. This reaction takes place all the time in any quantity of water.
If you have a sample of pure water, it means that the concentration of Hydronium ions and hydroxide ions is equal. Here is a demonstration in an equation .
In pure water: =
It is important to note that the process demonstrated in the equation above is easily reversible because water is a weak base and a weak acid. To establish the concentrations, it is important to look at the next concept of the autoionization constant.
Also Check: What Is Quantum Physics Mean
The Arrhenius Theory Of Acids And Bases
In 1884, the Swedish chemist Svante Arrhenius proposed two specific classifications of compounds acids and bases. When dissolved in an aqueous solution, certain ions were released into the solution. An Arrhenius acid is a compound that increases the concentration of H+ ions that are present when added to water. These H+ ions form the hydronium ion when they combine with water molecules. This process is represented in a chemical equation by adding H2O to the reactants side.
In this reaction, hydrochloric acid ) dissociates completely into hydrogen and chlorine ions when dissolved in water, thereby releasing H+ ions into solution. Formation of the hydronium ion equation:
The Arrhenius theory, which is the simplest and least general description of acids and bases, includes acids such as HClO4 and HBr and bases such as \ or \_2\). For example the complete dissociation of \ gas into water results generates free \ ions.
This theory successfully describes how acids and bases react with each other to make water and salts. However, it does not explain why some substances that do not contain hydroxide ions, such as \ and \, can make basic solutions in water. The Brønsted-Lowry definition of acids and bases addresses this problem.
An Arrhenius base is a compound that increases the concentration of OH- ions that are present when added to water. The dissociation is represented by the following equation:
Note
Predict Equilibrium And Strength Of Acids Using Ka And Pka
Ka can be used to calculate the equilibrium position:
- When Ka is big, the creation of dissociation products is encouraged.
- When Ka is low, the undissolved acid takes precedence.
Ka can be used to estimate an acids strength:
- If Ka is high , the acid is largely dissociated and therefore powerful. Strong acids have a pKa less than or equal to -2.
- When Ka is low , there has been little dissociation, hence the acid is weak. Weak acids have a pKa in water that ranges from -2 to 12.
Because adding water to an acid solution does not change its acid equilibrium constant, but it does modify the H+ ion concentration and pH, Ka is a better estimate of an acids strength than pH.
Read Also: What Does Inert Mean In Chemistry
Closer Look At Kw Autoionization Of Water
Need to know exactly what is the equation for autoionization of water? Autoionization constant is expressed as demonstrated in equation below.
Kw =
At this point, it is prudent to appreciate that when writing Kw constant chemistry expressions, you omit the concentrations. What does this imply when writing or working on Kw? It means that Kw, in this case, is a pure water sample.
Note that Kw in chemistry is dependent on temperature. For example, you can calculate the Kw chemistry equation value at 250C utilizing H3O+, which is closely related to water PH. At 25 degrees, the PH of pure water is 7. Therefore, what is the concentration of hydronium ions in such a sample? Here is a demonstration in equation :
At 250C =10pH=107 M
So, how to find Kw chemistry? Based on college homework helper experts to understand chemistry Kw, we know that the concentration of hydroxide and hydronium ions follows the ration of 1:1 in the autoionization of water equation. Therefore, we can advance equation above to calculate hydroxide ions, as demonstrated in equation below:
At 250C ==107 M
In many cases, students find it hard to visualize the entire reaction. However, 10-7 is a very small figure, and, therefore, only a relatively small quantity of water will be in the ionized form. From equation above, we can go ahead and calculate Kw of water at 250C. See the equation of how to calculate Kw chemistry below:
Kw=×=1014 at 25C
Overview Of Acids And Bases
There are three major classifications of substances known as acids or bases. The Arrhenius definition states that an acid produces H+ in solution and a base produces OH-. This theory was developed by Svante Arrhenius in 1883. Later, two more sophisticated and general theories were proposed. These are the Brønsted-Lowry and the Lewis definitions of acids and bases. The Lewis theory is discussed elsewhere.
Read Also: Carnegie Learning Algebra 2 Answer Key