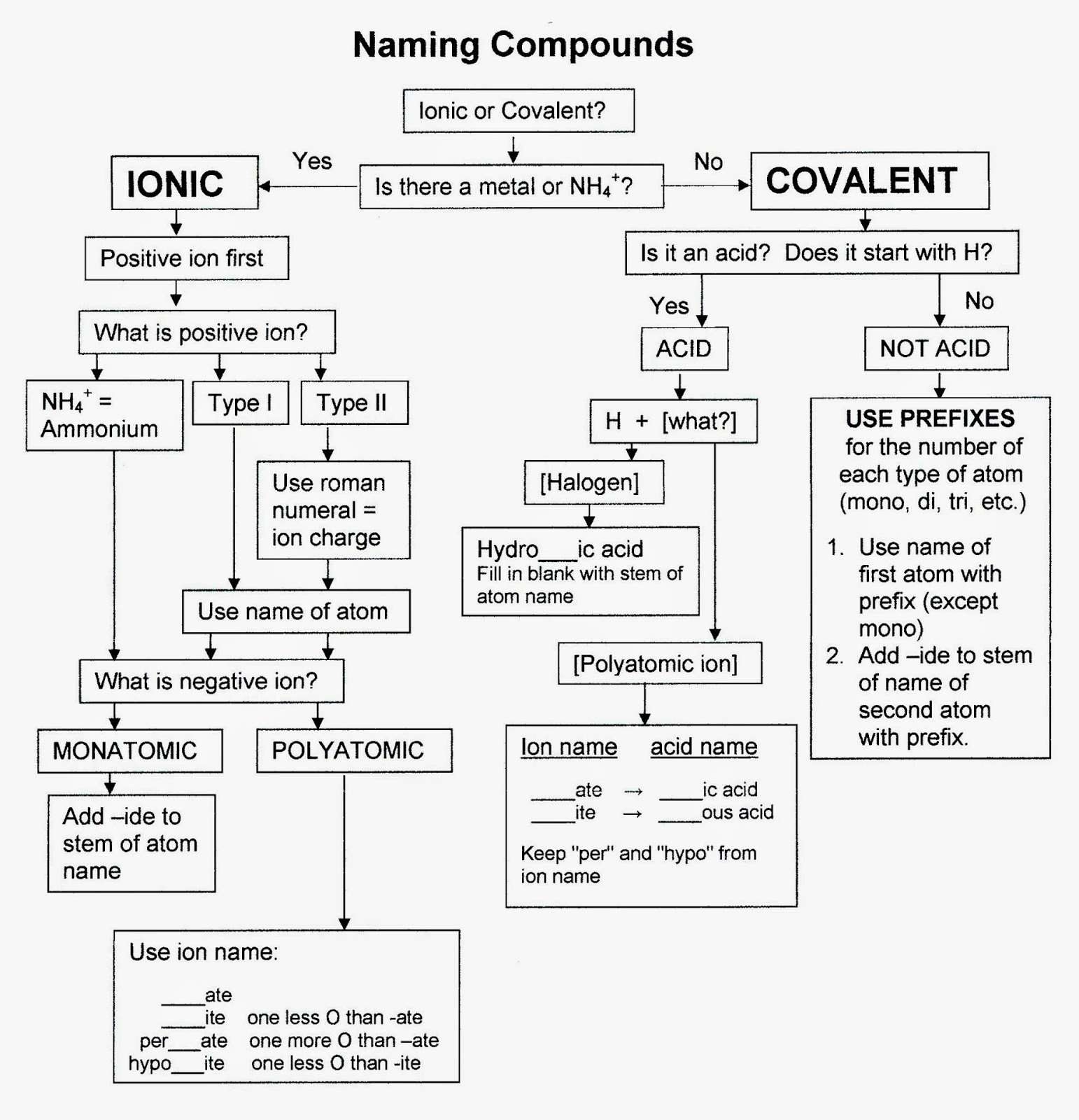
The Old Classic Or Common Way Of Naming
Names of some ionic compounds: Common, or trivial, names of compounds are sometimes used in informal conversations between chemists, especially older chemists. Systematic names are formal names that are always used in print.
Since some metallic elements form cations that have different positive charges, the names of ionic compounds derived from these elements must contain some indication of the cation charge. The older method uses the suffixes -ous and -ic to denote the lower and higher charges, respectively. In the cases of iron and copper, the Latin names of the elements are used . This system is still used, although it has been officially supplanted by the more precise, if slightly cumbersome, Stock system. In both systems, the name of the anion ends in -ide.
Naming Compounds Part 1 YouTube: This video explains how to name covalent and ionic compounds.
How Do You Name A Compound
Molecular compounds are named with the first element first and then the second element by using the stem of the element name plus the suffix -ide. Numerical prefixes are used to specify the number of atoms in a molecule.
Video advice: Naming Covalent Molecular Compounds
Well learn how to write names for compounds that are made of two nonmetals, sometimes called binary compounds. Binary compounds made of two nonmetals are called covalent or molecular because the elements are held together with covalent bonds, and they make molecules. In order to name them, we use the element name for the first element in the chemical formula, and then we use the -ide name for the second name in the chemical formula. Greek prefixes to show the number of atoms of each element, and these are put in front of the element names.
Erin Brokovich And Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr used by Pacific Gas & Electric to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brokovich , Erin and lawyer Edward Masry sued PG& E for contaminating the water near Hinckley in 1993. The settlement they won in 1996$333 millionwas the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Figure 1. Erin Brockovich found that Cr, used by PG& E, had contaminated the Hinckley, California, water supply. The Cr ion is often present in water as the polyatomic ions chromate, CrO42 , and dichromate, Cr2O72 .
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr or Cr forms. Cr, an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Also Check: Mcdougal Littell Geometry Workbook Answers
Naming Binary Covalent Compounds
There are many other naming schemes. Thereare naming schemes for acids, organic compounds and simple covalent compounds. You book covers simple covalent compounds in this chapter probablybecause it is so similar to the naming scheme for ionic compounds. Remember, ionic compounds are metal combined with a non-metal. A covalent compound is the combination of non-metals.
Rules for naming simple covalentcompounds:
1. Name the non-metal furthest to the left on the periodic tableby its elemental name.
2. Name the other non-metal by its elemental name and an -ideending.
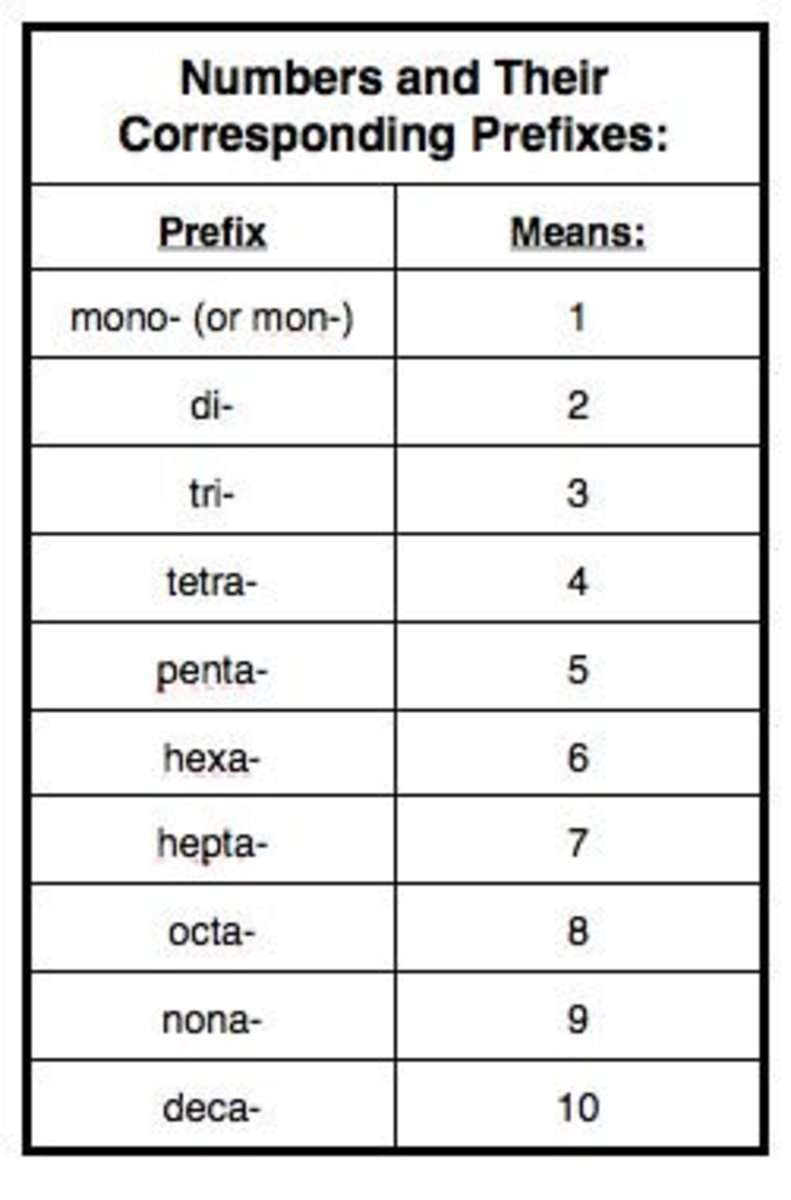
3. Use the prefixes mono-, di-, tri-…. to indicate the numberof that element in the molecule.
4. If mono is the first prefix, it is understood and not written
N2O4 iscalled dinitrogen monoxide
CO2 is called carbondioxide
CO is called carbon monoxide
N2O is called dinitrogenmonoxide.
CCl4 is called carbontetrachloride
Here is a chart of those prefixes:
|
1 – mono |
Finding Chemical Formulas From Names Of Chemical Compounds
We have discovered how to change chemical formulae into names of chemicals. What about converting compound names into compound formulas? The process is not much different. For ionic compounds, start by listing the component ions. Write the charges on the ions. Find the ratio of positive ions to negative ions that results in a net charge of zero. This is called the “criss-cross” or “crossover” method. For covalent compounds the process is simpler, as the ratio of elements is indicated by the numerical prefixes.
Examples:
Copper Nitrate = Cu
Carbon Dioxide = CO
Read Also: Mcdougal Littell Geometry Book Answers
Types Of Chemical Formulae
While the term chemical formula typically refers to the molecular formula of a compound , the compositions of chemical compounds can be expressed in several ways, as listed in this subsection.
Molecular Formula:
The molecular formula provides insight into the number of elements present in a compound. In molecular formulae, the elements are denoted by their respective symbols and the number of atoms of each element in the molecule is written in subscript. For example- the molecular formula for glucose is C6H12O6.
Empirical Formula:
The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Empirical formulae are usually obtained based on the analysis of experimental data. The empirical formula for glucose is CH2O. Empirical Formulae can be derived from the molecular formulae.
Structural Formula:
As the name suggests, the structural formula of a chemical compound provides insight into the arrangement of the atoms in the molecule.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Visit BYJUS for all Chemistry related queries and study materials
Your result is as below
How To Get A Systematic Name From A Structure
To assign a name to a compound, begin by determining the parent chain, which is the longest straight chain of carbon atoms. On paper, you should be able to put a finger down on one end of the parent chain and trace through all carbons until you get to the end, without needing to lift your finger. Well start with the simplest straight chain alkane structures.
If the parent chain is just one carbon long, the name is based on CH4 which is called methane. For a two-carbon parent chain the name will be based on C2H6, which is ethane. The table below continues with the names of longer straight-chain alkanes. While rote memorization is generally not the best way to learn organic chemistry, it may be worth committing these to memory, as they are the basis for the rest of the IUPAC nomenclature system. With some practice they will become part of your functional vocabulary.
9 carbons: nonane
10 carbons: decane
While many of these names share a Greek root with more familiar geometric shape names, some do not. For the first four, chemistry students often learn their order with the aid of the mnemonic Mice Eat Peanut Butter.
The structure shown below is laid out on the page so that the longest continual carbon chain is oriented vertically. Structures that are presented this way can be confusing, leading to misinterpretation. In this case the structure could be accidentally named 2-ethylpropane instead of 2-methylbutane .
Exercise 2.2.1
Exercise 2.2.2
a) methylcyclopentane
Also Check: Michael Jacksons Biological Kids
Naming Ionic Compounds Using Hypo
In the case where there is a series of four oxyanions, the hypo- and per- prefixes are used in conjunction with the -ite and -ate suffixes. The hypo- and per- prefixes indicate less oxygen and more oxygen, respectively.
- ClO- Hypochlorite
- ClO3- Chlorate
- ClO4- Perchlorate
Example: The bleaching agent sodium hypochlorite is NaClO. It is also sometimes called the sodium salt of hypochlorous acid.
Some Compounds Have Both Covalent And Ionic Bonds
If you recall the introduction of polyatomic ions, you will remember that the bonds that hold the polyatomic ions together are covalent bonds. Once the polyatomic ion is constructed with covalent bonds, it reacts with other substances as an ion. The bond between a polyatomic ion and another ion will be ionic. An example of this type of situation is in the compound sodium nitrate. Sodium nitrate is composed of a sodium ion and a nitrate ion. The nitrate ion is held together by covalent bonds and the nitrate ion is attached to the sodium ion by an ionic bond.
Recommended Reading: Algebra 1 Eoc Fsa Practice Test No Calculator Portion Answers
Erin Brockovich And Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr used by Pacific Gas & Electric to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brokovich , Erin and lawyer Edward Masry sued PG& E for contaminating the water near Hinckley in 1993. The settlement they won in 1996$333 millionwas the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Figure 1.
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr or Cr forms. Cr, an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Aims Of Chemical Nomenclature
The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to: each chemical name should refer to a single substance. A less important aim is to ensure that each substance has a single name, although a limited number of alternative names is acceptable in some cases.
Preferably, the name also conveys some information about the structure or chemistry of a compound. The American Chemical Society‘s CAS numbers form an extreme example of names that do not perform this function: each CAS number refers to a single compound but none contain information about the structure.
The form of nomenclature used depends on the audience to which it is addressed. As such, no single correct form exists, but rather there are different forms that are more or less appropriate in different circumstances.
A common name will often suffice to identify a chemical compound in a particular set of circumstances. To be more generally applicable, the name should indicate at least the chemical formula. To be more specific still, the three-dimensional arrangement of the atoms may need to be specified.
Don’t Miss: Is Paris Jackson Michael Jacksons Biological Daughter
Rules For Naming Molecular Compounds
- Write the name for both elements.
- Change the ending of the second element to ide.
- Place prefixes in front of each element based on the number of atoms present.
- The prefix ‘mono’ is only used on the second non-metal in the chemical formula.
- There shouldn’t be two vowels in a row. For example, when you have ‘mono’ in front of ‘oxide’ it is written ‘monoxide’, not ‘monooxide’.
Atomic Structure And Properties Relating To Bonding
Everything is made of atoms. Atoms themselves are made of smaller particles. Elements can join together in different ways to form compounds with different properties.
When elements combine or join together new substances are formed. These substances are called compounds.
This is shown in the diagram below
There are millions of different compounds and all of them have different properties. The properties of compounds are linked to the type of bonds formed within them.
You May Like: How To Do Percent Error Chemistry
Why Is Nomenclature Important What Is The Purpose Of Nomenclature
There are two objectives of using nomenclature in chemistry:
- To make sure that a spoken or written chemical name does not contain any ambiguity regarding the chemical compound the name is referring towards. It is important that each chemical name points towards a single substance.
- To ascertain that each substance has one name only
- To help the chemists communicate with their peers easily.
Emma
Rules For Naming Molecular Compounds:
Generally, the more electropositive atom is written first, followed by the more electronegative atom with an appropriate suffix. For example, H2O can be called dihydrogen monoxide . Organic molecules do not follow this rule.
Also Check: The Beth Thomas Story
How To Write Chemical Formula
In order to write a chemical formula, it is important to know the symbol of the elements present in the compound, formula of the radicals and the valency of the elements in that compound. Following points should be kept in mind while writing a chemical formula.
- Most of the compounds are binary compounds, i.e. they have two elements. Compounds with more than two elements are also known
- An atom with a positive charge is called a cation whereas an atom with a negative charge is called an anion
- For a compound containing a metal and a non-metal, the metal is named first followed by the non-metal. For example: NaCl which consists of Na+ and Cl
- Anions having -1 negative charge usually have a suffix as ide. For example: F Floride
- Anions having oxyanions usually have a suffix as ate. For example SO42-
- When a polyatomic anion has H ion, bi- or hydrogen is used as a suffix. For example HCO3-Bicarbonate or hydrogen carbonate
- Some polyatomic anions can be named as:
| Chemical Formula |
| Cyanide |
Solved Examples
Problem 1: In one molecule of the compound, determine how many atoms of every element are present for each one of these chemical formulas.
Answer:
Differing Aims Of Chemical Nomenclature And Lexicography
It is generally understood that the aims of lexicography versus chemical nomenclature vary and are to an extent at odds. Dictionaries of words, whether in traditional print or on the web, collect and report the meanings of words as their uses appear and change over time. For web dictionaries with limited or no formal editorial process, definitions âin this case, definitions of chemical names and termsâ can change rapidly without concern for the formal or historical meanings. Chemical nomenclature on the other hand is necessarily more restrictive: It aims to standardize communication and practice so that, when a chemical term is used it has a fixed meaning relating to chemical structure, thereby giving insights into chemical properties and derived molecular functions. These differing aims can have profound effects on valid understanding in chemistry, especially with regard to chemical classes that have achieved mass attention. Examples of the impact of these can be seen in considering the examples of:
Don’t Miss: Who Is Khloe Kardashian’s Real Father
Naming Ionic Compounds Using
Although Roman numerals are used to denote the ionic charge of cations, it is still common to see and use the endings -ous or -ic. These endings are added to the Latin name of the element to represent the ions with lesser or greater charge, respectively. The Roman numeral naming convention has wider appeal because many ions have more than two valences.
- Fe2+ Ferrous
Example: FeCl3 is ferric chloride or iron chloride.
How To Name Binary Acids And Oxyacids In General Chemistry
Acids are a type of covalent compound that release hydrogen ions when dissolved in water. Binary acids are composed of hydrogen and one other element, while oxyacids contain hydrogen, oxygen, and another element. Translating binary acids from formula to name will end up in the format hydroic acid. Translating oxyacids from formula to name involves dropping the word hydrogen completely, replacing the anions suffix, and adding acid. The oxyacid suffix replacements are -ate becomes -ic and -ite becomes -ous.
Note that the definition of acid includes dissolved in water. That means all acids, binary and oxyacid, will be aqueous, or dissolved in water. This will be marked by the subscript “aq,” as shown in the examples below.
Examples:
H2CO3 = Carbonic Acid
Recommended Reading: Glencoe Mcgraw Hill Geometry Concepts And Applications Answers
Compounds Containing A Metal Ion With A Variable Charge
Most of the transition metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ , and the two corresponding compound formulas are FeCl2 and FeCl3. The simplest name, iron chloride, will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron chloride and iron chloride, respectively. Other examples are provided in Table 4.
| Table 4. Names of Some Transition Metal Ionic Compounds | |
|---|---|
| Transition Metal Ionic Compound | |
| SnF4 | tin flouride |