How Do I Calculate The Excess Electrons Number
To calculate the excess of electrons, you need only to know the charge of your object. You can measure this quantity in various ways, even though the instrument is always called an electrometer. Once you know this quantity, in coulombs, apply the formula to calculate the excess electrons:
- ne Calculated number of excess electrons
- Q Q Charge of the object and
- e e Electron charge.
Calculating Electric Flux With Charge
The electric flux through a planar area A of an electric field E is the field multiplied by the component of the area perpendicular to the field. To get this perpendicular component, you use the cosine of the angle between the field and the plane of interest in the formula for flux, represented by = EA cos, where is the angle between the line perpendicular to the area and the direction of the electric field.
This equation, known as Gauss’s Law, also tells you that, for surfaces like these ones, which you call Gaussian surfaces, any net charge would reside on its surface of the plane because it would be necessary to create the electric field.
Because this depends on the geometry of the area of the surface used in calculating flux, it varies depending on the shape. For a circular area, the flux area A would be _r_2with r as the radius of the circle, or for the curved surface of a cylinder, the flux area would be Ch in which C is the circumference of the circular cylinder face and h is the cylinder’s height.
Conduction Of Electricity And Heat
The free-electron collisions transfer energy to the atoms of the conductor. The electric field does work in moving the electrons through a distance, but that work does not increase the kinetic energy of the electrons. The work is transferred to the conductors atoms, possibly increasing temperature. Thus a continuous power input is required to keep a current flowing. An exception, of course, is found in superconductors, for reasons we shall explore in a later chapter. Superconductors can have a steady current without a continual supply of energya great energy savings. In contrast, the supply of energy can be useful, such as in a lightbulb filament. The supply of energy is necessary to increase the temperature of the tungsten filament, so that the filament glows.
Read Also: Which Founding Contributors To Psychology Helped Develop Behaviorism
How Do I Calculate The Excess Or Deficit Number Of Electrons
To calculate from coulomb to excess of electrons , you need to follow a few simple steps:
Write down the charge of an electron:e = 1.602176634 × 1019
Measure the charge Q of the desired object. You can do it with an electrometer.
Divide Q by the charge of a single electron. The result is the number of electrons :
n = Q/e
Notice that a negative charge corresponds to an excess of electrons only if you consider e to be negative.
Example 3 Calculating Drift Velocity In A Common Wire
Calculate the drift velocity of electrons in a 12-gauge copper wire carrying a 20.0-A current, given that there is one free electron per copper atom. The density of copper is 8.80 × 103 kg/m3.
Strategy
We can calculate the drift velocity using the equation I = nqAvd. The current I = 20.0 A is given, and q = 1.60×1019 C is the charge of an electron. We can calculate the area of a cross-section of the wire using the formula A = r2 , where r is one-half the given diameter, 2.053 mm. We are given the density of copper, 8.80 × 103 kg/m3 and the periodic table shows that the atomic mass of copper is 63.54 g/mol. We can use these two quantities along with Avogadros number, 6.02 × 1023 atoms/mol, to determine n, the number of free electrons per cubic meter.
Solution
First, calculate the density of free electrons in copper. There is one free electron per copper atom. Therefore, is the same as the number of copper atoms per m3. We can now find n as follows:
\beginn& =& \frac^}}\times \frac\text\times }^}\text}}\times \frac}\text\text}\times \frac}}\times \frac\times }^\text}}^}\\ & =& \text\text\text\times }^}^}^\end\\.
The cross-sectional area of the wire is
\beginA& =& \pi ^\\ & =& \pi }^\text}\right)}^\\ & =& \text\times }^}}^\text\end\\
Rearranging I = nqAvd to isolate drift velocity gives
\begin_}=\frac}}\\ =\frac}\text\times }^}}^\right)\left\left}\\ =\text\text\text\times }^}\text\end\\
Discussion
Don’t Miss: What Are The 3 Undefined Terms In Geometry
A Polythene Piece Rubbed With Wool Is Found To Have A Negative Charge Of 3 107 C Estimate The Number Of Electrons Transferred
A polythene piece rubbed with wool is found to have a negative charge of 3 × 107 C.
Estimate the number of electrons transferred
Is there a transfer of mass from wool to polythene?
SolutionShow Solution
When polythene is rubbed against wool, a number of electrons get transferred from wool to polythene. Hence, wool becomes positively charged and polythene becomes negatively charged.
Amount of charge on the polythene piece, q = 3 × 107 C
Amount of charge on an electron, e = 1.6 × 1019 C
Number of electrons transferred from wool to polythene = n
n can be calculated using the relation,
q = ne
Conservation Of Electric Charge
If a system remains isolated , it will conserve charge. Conservation of charge means that the total amount of electric charge remains the same for the system. Conservation of charge lets physicists and engineers calculate how much charge moves between systems and their surroundings.
This principle lets scientists and engineers create Faraday cages that use metallic shields or coating to prevent charge from escaping. Faraday cages or Faraday shields use an electric field’s tendency to re-distribute charges within the material to cancel out the effect of the field and prevent the charges from harming or entering the interior. These are used in medical equipment such as magnetic resonance imaging machines, to prevent data from being distorted, and in protective gear for electricians and linemen working in hazardous environments.
You can calculate the net charge flow for a volume of space by calculating the total amount of charge entering and subtracting the total amount of charge leaving. Through electrons and protons that carry charge, charged particles can be created or destroyed to balance themselves out according to conservation of charge.
Recommended Reading: What Are The Biological Causes Of Depression
How To Calculate Electrical Charge
Whether it’s static electricity given off by a furry coat or the electricity that powers television sets, you can learn more about electric charge by understanding the underlying physics. The methods to calculate charge depend on the nature of electricity itself, such as principles of of how charge distributes itself through objects. These principles are the same no matter where you are in the universe, making electrical charge a fundamental property of science itself.
Gauss’s Law In Other Situations
Because the net charge on a surface must remain in electrostatic equilibrium, any electric field must be perpendicular to the surface of a conductor to allow the material to transmit charges. Gauss’s law lets you calculate the magnitude of this electric field and flux for the conductor. The electric field inside a conductor must be zero, and, outside, it must be perpendicular to the surface.
This means, for a cylindrical conductor with field radiating from the walls at a perpendicular angle, the total flux is simply 2_E__r_2 for an electric field E and r radius of the circular face of the cylindrical conductor. You can also describe the net charge on the surface using , the charge density per unit area, multiplied by the area.
Related Articles
Recommended Reading: What Is O2 In Chemistry
Calculating Electric Charge In Circuits
If you know the electric current, the flow of electric charge through an object, traveling through a circuit and how long the current is applied, you can calculate electrical charge using the equation for current Q = It in which Q is the total charge measured in coulombs, I is current in amps, and t is time that the current is applied in seconds. You can also use Ohm’s law to calculate current from voltage and resistance.
For a circuit with voltage 3 V and resistance 5 that is applied for 10 seconds, the corresponding current that results is I = V / R = 3 V / 5 = 0.6 A, and the total charge would be Q = It = 0.6 A × 10 s = 6 C.
If you know the potential difference in volts applied in a circuit and the work in joules done over the period which it is applied, the charge in coulombs, Q = W / V.
Why Does My Fleece Blanket Spark
The rubbing between your body and the blanket causes many electrons to be transferred, thus causing the development of a relatively high excess charge on the blanket and an equal charge with the opposite sign on your body.
If you get close enough to the blanket with an exposed part of your body, the electrons you exchange with the blanket will return to the original object through a spark. But only if the potential difference between the two parts is high enough to ionize the air between them.
You May Like: Is Paris Jackson Michael’s Biological Child
Electric Potential And Potential Difference
- Define electric potential, voltage, and potential difference
- Define the electron-volt
- Calculate electric potential and potential difference from potential energy and electric field
- Describe systems in which the electron-volt is a useful unit
- Apply conservation of energy to electric systems
Recall that earlier we defined electric field to be a quantity independent of the test charge in a given system, which would nonetheless allow us to calculate the force that would result on an arbitrary test charge. We briefly defined a field for gravity, but gravity is always attractive, whereas the electric force can be either attractive or repulsive. Therefore, although potential energy is perfectly adequate in a gravitational system, it is convenient to define a quantity that allows us to calculate the work on a charge independent of the magnitude of the charge. Calculating the work directly may be difficult, since \ and the direction and magnitude of \ can be complex for multiple charges, for odd-shaped objects, and along arbitrary paths. But we do know that because \, the work, and hence \ is proportional to the test charge \. To have a physical quantity that is independent of test charge, we define electric potential \ to be the potential energy per unit charge:
Electric Potential
The electric potential energy per unit charge is
Electric Potential Difference
Potential Difference and Electrical Potential Energy
Example \: Calculating Energy
Strategy
Solution
Example 1 Calculating Currents: Current In A Truck Battery And A Handheld Calculator
What is the current involved when a truck battery sets in motion 720 C of charge in 4.00 s while starting an engine? How long does it take 1.00 C of charge to flow through a handheld calculator if a 0.300-mA current is flowing?
Strategy
We can use the definition of current in the equation I = Q/t to find the current in part , since charge and time are given. In part , we rearrange the definition of current and use the given values of charge and current to find the time required.
Solution for
Entering the given values for charge and time into the definition of current gives
\beginI& =& \frac=\frac}}=\text\\ & =& \text\end\\
Discussion for
This large value for current illustrates the fact that a large charge is moved in a small amount of time. The currents in these starter motors are fairly large because large frictional forces need to be overcome when setting something in motion.
Solution for
Solving the relationship I = Q/t for time t, and entering the known values for charge and current gives
\begin\Delta t& =& \frac=\frac}}^\text}\\ & =& \text\times }^\text\end\\
Discussion for
Figure 2. A simple electric circuit. A closed path for current to flow through is supplied by conducting wires connecting a load to the terminals of a battery. In this schematic, the battery is represented by the two parallel red lines, conducting wires are shown as straight lines, and the zigzag represents the load. The schematic represents a wide variety of similar circuits.
Don’t Miss: What Is Parsimony In Biology
Before Calculating Excess Electrons: Electrons And The Charge Of An Object
Have you ever got a spark from one of your friends? No, not in that way we are talking of physics! Sparks are a consequence of the accumulation of charge on an object.
But… what is charge? Charge short for electric charge is a property of matter that describes how much an object feels the effects of an electric field. This definition may be unsatisfactory, but we know: charge is a fundamental property, as is mass, and this makes it pretty hard to find a way to define it without using some degree of redundancy or leaving something “untold”.
Even though the charge may be a bit mysterious, its origin is straightforward to explain: we are talking of electrons. Electrons are elementary subatomic particles, which means that we can’t break them into smaller pieces, carrying what we call unitary charge. Scientists are pretty sure that the charge of an electron is the smallest one that can live “free”.
In 2019, scientists fixed the value of the electron charge. Now, at least for physicists, the electron charge is something we use to measure things and not something we measure. This is its value:
We measure this quantity in coulombs, the measurement unit for charge.
Every electric charge in the universe is an integer multiple of the charge of an electron this makes the calculations for the excess electrons’ number easy matter, if not for a small detail: the charge of an electron is incredibly small!
How Many Moles Of Electrons Are Transferred When One Mole Of Cu Is Formed
The moles of electrons used = 2 x moles of Cu deposited. Moles of Cu deposited = 1.00 / 63.55 = 1.574 x 10-2 mol, so moles of electrons passed = 2 x 1.574 x 10-2 = 3.148 x 10-2 mol. From the stoichiometry of this equation, one mole of Na deposited requires the passage of one mole of electrons in the electrolysis.
You May Like: What Is The Formula For Distance In Physics
Example 2 Calculating The Number Of Electrons That Move Through A Calculator
If the 0.300-mA current through the calculator mentioned in Example 1 is carried by electrons, how many electrons per second pass through it?
Strategy
The current calculated in the previous example was defined for the flow of positive charge. For electrons, the magnitude is the same, but the sign is opposite, Ielectrons = 0.300 × 103C/s. Since each electron has a charge of 1.60×1019 C, we can convert the current in coulombs per second to electrons per second.
Solution
Starting with the definition of current, we have
_}=\frac_}}=\frac\times }^\text}}\\
We divide this by the charge per electron, so that
\begin\frac^}}}& =& \frac\times }^\text}}\times \frac^}}\times }^}\text}\\ & =& \text\times }^}\frac^}}}\end\\.
Discussion
There are so many charged particles moving, even in small currents, that individual charges are not noticed, just as individual water molecules are not noticed in water flow. Even more amazing is that they do not always keep moving forward like soldiers in a parade. Rather they are like a crowd of people with movement in different directions but a general trend to move forward. There are lots of collisions with atoms in the metal wire and, of course, with other electrons.
Electric Charge And Gravity: Similarities
Coulomb’s law bears striking similarity to Newton’s law for gravitational force FG = G m1m2 / r2 for gravitational force FG, masses m1and m2, and gravitational constant G = 6.674 × 10 11 m3/ kg s2. They both measure different forces, vary with greater mass or charge and depend upon the radius between both objects to the second power. Despite the similarities, it’s important to remember gravitational forces are always attractive while electric forces can be attractive or repulsive.
You should also note that the electric force is generally much stronger than gravity based on the differences in the exponential power of the constants of the laws. The similarities between these two laws are a greater indication of symmetry and patterns among common laws of the universe.
Read Also: What Is Symmetry In Biology
What Is The Consequence Of An Excess Or Deficit Of Electrons
An excess or deficit of electrons causes an object to be charged. A charged object displays many properties and is subjected to phenomena different than the ones relative to neutral bodies.
-
A charged object feels the effect of an electric field, either being attracted or repelled by it.
-
A charged object can emit sparks if the charge is high enough to “break” the surrounding insulator.
A small shock is one of the ways you can perceive an excess of electrons!
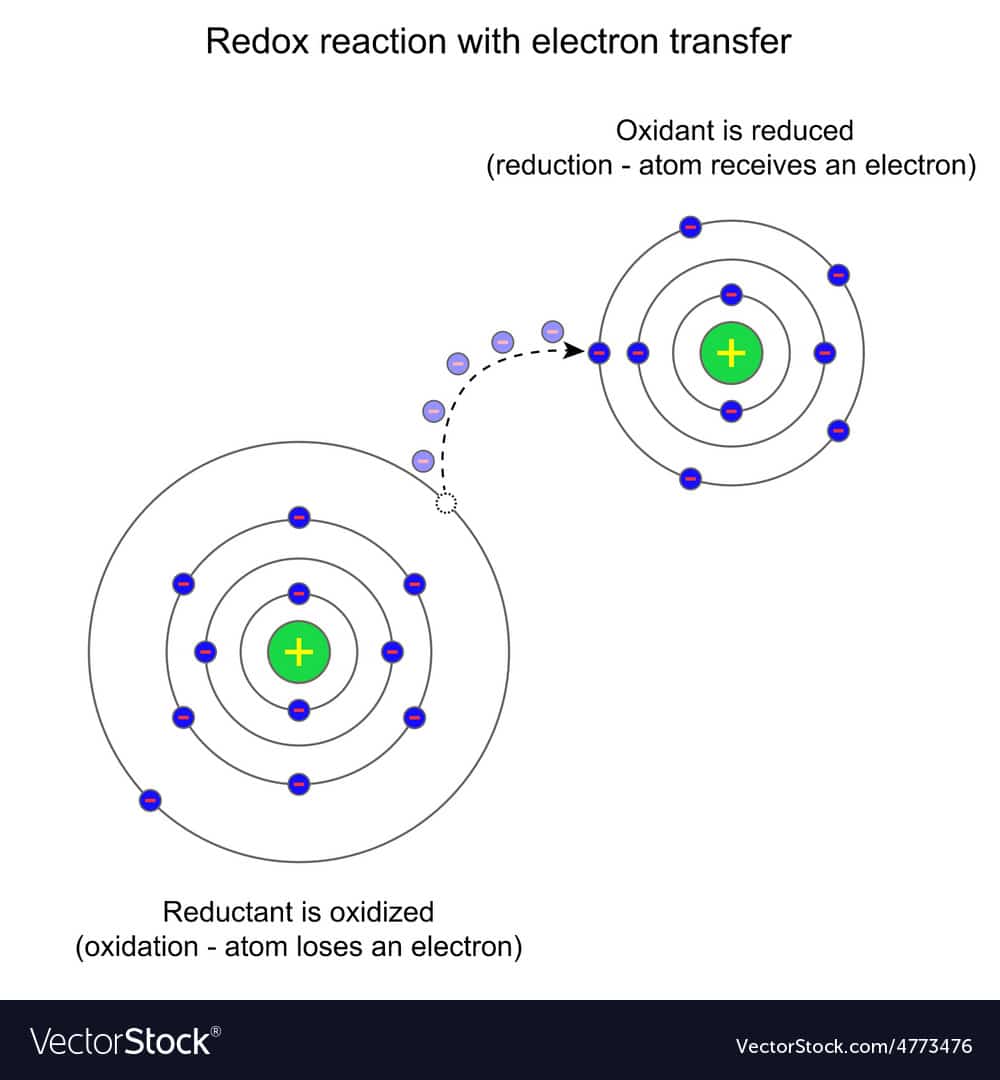
Determining Number Of Electrons Transferred
When determining the number of electrons transferred in a redox reaction is it the total in both half equations?
For example:
$$\ce$$
First we split it up into the two half-reactions and get the following
$$\ce$$
$$\ce$$
So would the number of electrons transferred in the redox reaction be two or four?
- 2Jul 23, 2015 at 23:28
- 2$\begingroup$@ToddMinehardt – While I think that the answer to the question you link will answer this question, the questions represent two very different misunderstandings. The question you link to is asking why ions have the charges they do.$\endgroup$
When determining the number of electrons transferred in a redox reaction is it the total in both half equations?
In the total redox reaction that is properly balanced, the number of electrons transferred is equal to the stoichiometric coefficient of the $\ce$ species in either balanced half-reaction.
So would the number of electrons transferred in the redox reaction be two or four?
Your half-reactions and total redox reaction happen to be balanced, to the number of electrons transferred in this case is two.
Also Check: How Did Geography Of Rome Affect Its Expansion
Charge Current And Voltage
Electrical current transfers energy around circuits. There are two types of current: direct and alternating.
An electric current is a flow of charged particles.
In metal conductors the charged particles are free electrons.
The electrons are free to move from one ion to another and a net flow of these electrons in one direction is an electric current.
A source of energy, such as a cell or battery, is required to make the free electrons move in one direction.