How Is Soap Made
Soap was reportedly first invented around 2800 BCE, by the ancient Babylonians. The first soaps were made out of a conglomeration of water, animal fat and wood ash, and were mainly used to wash wool and cotton. The process has been refined and the ingredients have changed a little over thousands of years, but soap remains largely the same in its formula and application.
Soap is created through the combination of alkali, fats and oils. The alkalis in todays soaps are usually derived from substances like lye or caustic potash, though they were traditionally made out of ash from wood. The oils found in soap are usually plant-based, like coconut oil, and fats usually come from animal sources like beef tallow.
A process integral to the creation of soap is referred to as saponification, and it involves using the alkali to divide the oils and fats into glycerin and fatty acids respectively. The potassium or sodium found from the alkali is then able to join the fatty acids within these compounds and, as a byproduct, water and soap are produced.
How Do You Make Soap Project Chemistry
Heat 50 mL of saturated sodium chloride solution in a 100-mL beaker until it is almost boiling.
Propelled Viruses And The Surfaces They Hit
When you cough, or especially when you sneeze, tiny droplets from the airways can fly up to 10 meters ! The larger ones are thought to be main coronavirus carriers and they can go at least 2 m . Thus cover your coughs & sneezes people!
These tiny droplets end on surfaces and often dry out quickly. But the viruses are still active! What happens next is all about supramolecular chemistry and how self-assembled nanoparticles interact with their environment!
Now it is time to introduce a powerful supramolecular chemistry concept that effectively says: similar molecules appear to interact more strongly with each other than dissimilar ones. Wood, fabric and not to mention skin interact fairly strongly with viruses.
Contrast this with steel, porcelain and at least some plastics, e.g. Teflon. The surface structure also matter the flatter the surface the less the virus will stick to the surface. Rougher surfaces can actually pull the virus apart.
So why are surfaces different? The virus is held together by a combination of hydrogen bonds and what we call hydrophilic or fat-like interactions. The surface of fibres or wood, for instance, can form a lot of hydrogen bonds with the virus.
In contrast steel, porcelain or Teflon do not form a lot of hydrogen bond with the virus. So the virus is not strongly bound to these surfaces. The virus is quite stable on these surface whereas it doesnt stay active for as long on say fabric or wood.
You May Like: Beth From Child Of Rage Now
Is Antibacterial Soap Even Better Nope
Antibacterial soaps have added ingredients like triclosan or triclocarban, which are hydrophobic molecules that can penetrate bacterial cell membranes and kill the bacteria. Sounds impressive, but studies have shown that antibacterial soaps are no more effective than regular soaps at removing bacteria.
In 2016, the FDA issued a rule that antibacterial soaps were no longer allowed to be marketed to the public.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” Dr. Janet Woodcock, the director of the FDA’s Center for Drug Evaluation and Research , said in a statement. “In fact, some data suggests that antibacterial ingredients may do more harm than good over the long term.”
The Disadvantage Of Soap
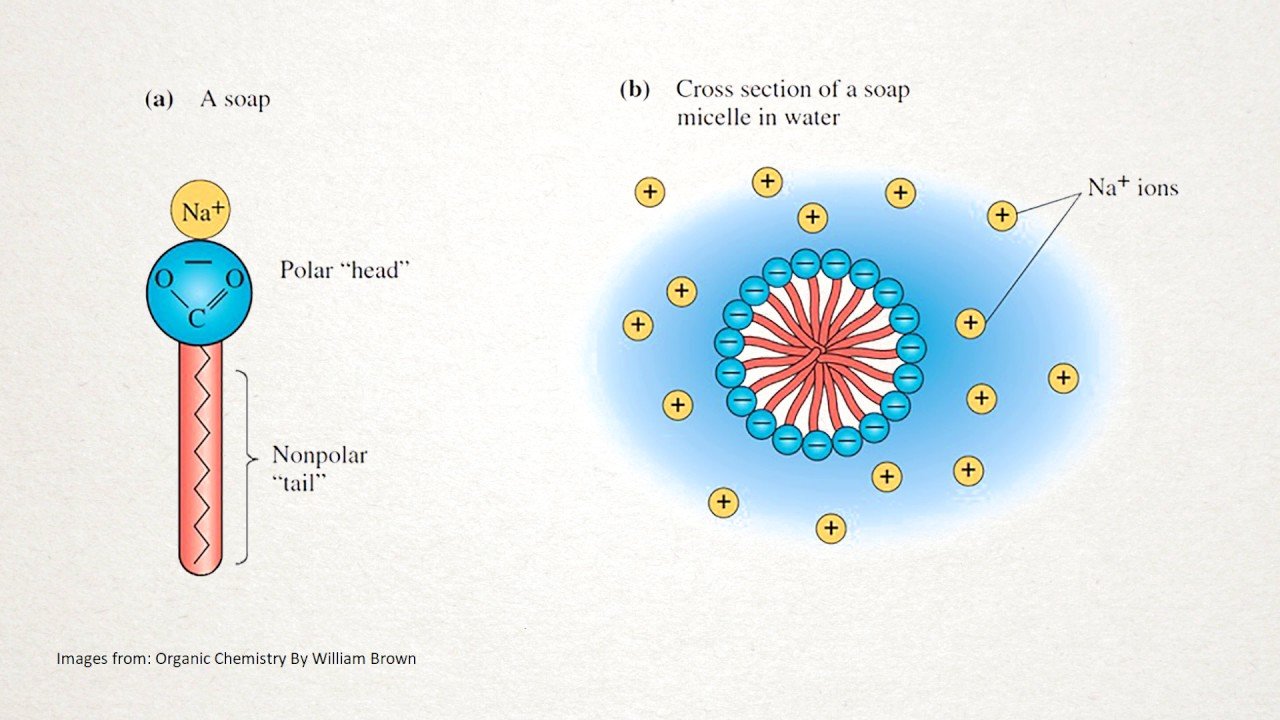
Although soaps are excellent cleansers, they do have disadvantages. As salts of weak acids, they are converted by mineral acids into free fatty acids:
CH316CO2-Na++ HCl â CH316CO2H + Na++ Cl-
These fatty acids are less soluble than the sodium or potassium salts and form a precipitate or soap scum. Because of this, soaps are ineffective in acidic water. Also, soaps form insoluble salts in hard water, such as water containing magnesium, calcium, or iron.
2 CH316CO2-Na++ Mg2+ â 2Mg2++ 2 Na+
The insoluble salts form bathtub rings, leave films that reduce hair luster, and gray/roughen textiles after repeated washings. Synthetic detergents, however, may be soluble in both acidic and alkaline solutions and don’t form insoluble precipitates in hard water. But that is a different story…
You May Like: Algebra 2 Regents 2016
Why Does Soap Work So Well On Sars
Posted onAuthorIan M Mackay, PhD
This is a guest post from Prof Palli Thordarson of the Uni of New South Wales. It was previously posted in a and on and has been reprinted here with the authors kind permission and with some light editing and formatting by VDUs Editor in Chief.
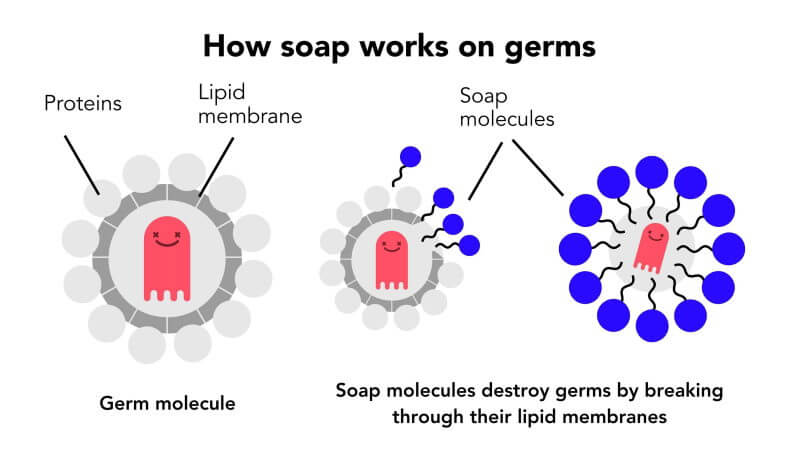
Why does soap work so well on the SARS-CoV-2, the coronavirus and indeed most viruses? Because it is a self-assembled nanoparticle in which the weakest link is the lipid bilayer.
The soap dissolves the fat membrane and the virus falls apart like a house of cards and dies, or rather, we should say it becomes inactive as viruses arent really alive. Viruses can be active outside the body for hours, even days.
Disinfectants or liquids, wipes, gels and creams containing alcohol have similar effects but are not really quite as good as normal soap. Apart from the alcohol and soap, the antibacterial agents in these products dont affect the virus structure much at all. Consequently, many antibacterial products are basically just an expensive version of soap in terms of how they act on viruses. Soap is the best but alcohol wipes are good when soap is not practical or handy .
I point out to that while I am expert in supramolecular chemistry and the assembly of nanoparticles, I am not a virologist. The first image here is from an excellent post here which is dense with good virology info:
Soap Not Handy Alcohol To The Rescue
Alcohol-based products, which pretty includes all disinfectants and antibacterial products contain a high-% alcohol solution, typically 60-80% ethanol, sometimes with a bit of isopropanol as well and then water + a bit of soap.
Ethanol and other alcohols do not only readily form hydrogen bonds with the virus material but as a solvent, are more lipophilic than water. Hence alcohol does also dissolve the lipid membrane and disrupt other supramolecular interactions in the virus.
However, you need a fairly high concentration of the alcohol to get a rapid dissolution of the virus. Vodka or whiskey , will not dissolve the virus as quickly. Overall alcohol is not quite as good as soap at this task.
Nearly all antibacterial products contain alcohol and some soap and this does help killing viruses. But some also include active bacterial killing agents, like triclosan. Those, however, do basically nothing to the virus!
Read Also: Holt Geometry Chapter 7 Test Form C Answers
How Soap Kills Covid
How 20 seconds of washing yours hands with soap will save lives, the chemistry explained:
Water alone may rinse off dirt, but viruses and bacteria are so small they often need chemical and mechanical intervention to get their sticky nanoparticles out of the crevices that make up our unique fingerprints. Thats why soap is so important. Its made for this job. Give soap 20 seconds, at least, of thorough scrubbing and the pin-shaped molecules will penetrate the types of bacteria and viruses, including COVID-19, that protect themselves with an oily lipid membrane. Like a nail popping a tire, the water-repelling end of the soap molecule, a hydrophobic tail that can bond with oil and fats, stabs COVID-19 and leaves the virus a deflated and broken sack of RNA cells.
And while alcohol can also break an oily membrane, washing with soap has the added benefit of physically removing even tougher to break viruses and bacteria from the skin. This is thanks to the dual nature of soap molecules. As the hydrophilic, or water-loving, heads reach out to bond with the water, the tails turn inwards to protect themselves from the water and by doing so, scoop up anything they catch in tiny soap bubble cages called micelles. Scrubbing all parts of your hands and wrists vigorously, with a sudsy lather, is key to locking these invading particles away for good – and washing them down the drain. And whether the water is cold or warm doesnt matter, so long as its soapy.
Images:
Soap Making: Fat Plus Lye Forms A Soap Plus Glycerol
The earliest recorded evidence of the production of soap-like materials dates back to around 2800 BC in ancient Babylon. A formula for soap consisting of water, alkali, and cassia oil was written on a Babylonian clay tablet around 2200 BC.
The Ebers papyrus indicates the ancient Egyptians bathed regularly and combined animal and vegetable oils with alkaline salts to create a soap-like substance. Egyptian documents mention a similar substance was used in the preparation of wool for weaving.In the reign of Nabonidus , a recipe for soap consisted of uhulu , cypress and sesame “for washing the stones for the servant girls”.In ancient Israel, the ashes from barilla plants, such as species of Salsola, saltwort and Anabasis, were used in soap production, known as potash
Figure \ Advertising for medicated toilet soap. Source: Wikipedia
Liquid soap was not invented until the nineteenth century in 1865, William Shepphard patented a liquid version of soap. In 1898, B.J. Johnson developed a soap derived from palm and olive oils his company, the B.J. Johnson Soap Company, introduced “Palmolive” brand soap that same year. This new brand of soap became popular rapidly, and to such a degree that B.J. Johnson Soap Company changed its name to Palmolive.
Figure \ Saponification.
You May Like: Abiotic Definition And Examples
The Chemistry Of Soap
Show of hands: who has become infinitely, intimately more familiar with soap recently? If 2020 was the year we learnt to wash meticulously in between our fingers and all the way up our arms, it was also the year we discovered just how long 20 seconds in front of a bathroom mirror can be.
But have you ever stopped to wonder how the humble bar of hand soap actually works? How does that slippery cake invented thousands of years ago remain on par with hand sanitiser or any of the other modern-day cleaning products we wield against a virus thats become a pandemic?
At its most basic, hand soap is just a combination of fat or oil with an alkaline substance. The first people we know of to lather up like this were the ancient Mesopotamians, who mixed animal fat with water and wood ash to produce a substance that, while no doubt greasy, smelly and hugely unpleasant, could also spirit away dirt and grime in a manner that must have appeared borderline miraculous.
At its most basic, the wood ash splits the animal fat or oil into molecules called amphiphiles. These amphiphiles have one end that loves water, and another that hates it.
One end is usually bulky and shorter we say its hydrophilic it interacts strongly with water, explains University of NSW chemistry professor Pall Thordarson. Then theres a longer hydrophobic end we call it a greasy tail.
Imagine you could see down to a molecular level while washing your hands.
What Harm Do Detergent Chemicals Do
You might think this is a matter of opinion mostly it’s a matter of science: the effects of detergent chemicals are welldocumented. What’s less well understood is that all chemicals are added to detergents for a specific purpose, and some of the additives actually reduce the harmful impacts that detergents would otherwise have.
Surfactants
As we’ve already seen, these play a crucial part in helping water to attack and remove dirt.But once they flush away down the drain, surfactants don’t stop working: they start to play similar trickson aquatic life, for example, attacking the natural oils in the mucus membranes of fish, stopping their gills from working properly, and increasing their risk of attack from other chemicalsin the water. Some surfactant ingredients produce what are calledendocrine-disruptors, which can affect the hormonal balance of animals . Although surfactants can be toxic to fish and other aquatic life ones that remain in the environment for many years without breaking down), most surfactants biodegrade relatively quickly in sewage treatment plants before they can domuch harm to the natural world.
Phosphates
Enzymes
Perfumes
Fragrances in detergent serve no purpose other than to make your clothes smell nice. But the oils from which they’re made cancause rashes and skin allergies.
Don’t Miss: June 2018 Algebra 2 Regents Answers
Saponification Of Oil Paintings
Sometimes the saponification reaction occurs unintentionally. Oil paint came into use because it withstood the test of time. Yet, over time the saponification reaction has led to damage of many oil paintings made in the fifteenth through twentieth centuries.
The reaction occurs when heavy metal salts, such as those in red lead, zinc white, and lead white, react with the fatty acids in the oil. The metal soaps produced by the reaction tend to migrate toward the surface of the painting, causing the surface to deform and producing a chalky discoloration called “bloom” or “efflorescence.” While a chemical analysis may be able to identify saponification before it becomes apparent, once the process starts, there is no cure. The only effective restoration method is retouching.
When The Surface Is Your Skin
The skin is an ideal surface for a virus! It is organic and the proteins and fatty acids in the dead cells on the surface interact with the virus through both hydrogen bonds and the fat-like hydrophilic interactions.
So when you touch say a steel surface with a virus particle on it, it will stick to your skin and hence get transferred onto your hands. But you are not infected. If you touch your face though, the virus can get transferred from your hands and on to your face.
And now the virus is dangerously close to the airways and the mucus type membranes in and around your mouth and eyes. So the virus can get inand voila! You are infected .
If the virus is on your hands you can pass it on by shaking someones else hand. Kisses, well, thats pretty obviousIt comes without saying that if someone sneezes right in your face you are kind of stuffed.
So how often do you touch your face? It turns out most people touch the face once every 2-5 minutes! Yeah, so you at high risk once the virus gets on your hands unless you can wash the active virus off.
You May Like: Cpt Code For Psychological Testing
What About Hand Sanitizer
The CDC recommends cleaning hands with soap and water, but if that’s not an option, then hand sanitizer is a good backup. Studies have found that hand sanitizers with alcohol concentrations of 60-95% are more effective at killing germs than nonalcohol or low-alcohol sanitizers.
Related: Hand sanitizer sold out? Here’s how to make your own.
The alcohol kills some bacteria and viruses by breaking down their protective membranes, which basically makes them fall apart. But it doesn’t work for all germs, such as norovirus, Clostridium difficile, which can cause life-threatening diarrhea, or Cryptosporidium, a parasite that causes a diarrheal disease called cryptosporidiosis, the CDC says. Hand sanitizers also likely don’t remove harmful chemicals like pesticides or heavy metals, nor does hand sanitizer work well on super dirty or greasy hands.
Hand washing with soap is, by far, the most effective way to keep harmful germs at bay.
Additional resources:
Disadvantages And Advantages Of Soap
The importance of soap to human civilization is documented by history, but some problems associated with its use have been recognized. One of these is caused by the weak acidity of the fatty acids. Solutions of alkali metal soaps are slightly alkaline due to hydrolysis. If the pH of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and magnesium salts in the water supply . These divalent cations cause aggregation of the micelles, which then deposit as a dirty scum.
In the reaction below, the sodium cation in soap is replaced by calcium to form calcium stearate. The white precipitate, also termed as soap scum could form deposits on surfaces and inside plumbing.
2 C17H35COONa+ + Ca2+ 2Ca + 2 Na+
Soap is still widely popular product as it is a low cost, readily available product used for personal hygiene and cleanliness. Use of soap doesn’t lead to pollution. Soap is biodegradable as it can be broken down by microorganisms found in sewage.
Can Soap Really Kill the Coronavirus ?
Video \ Soap and coronavirus.
Read Also: Simplifying Radicals Imaginary Numbers Worksheet Kuta Software
What About Antibacterial Soap
Soap is so good at removing substances from your hands that it can also wash away microbes and bacteria. Soap is able to kill and remove bacteria so effectively that there may not be much point to using soap with antibacterial agents included in them. A study done by the University of California found that antibacterial soaps are typically created by just infusing regular soap with compounds like triclocarban or triclosan. These compounds are used in toothpaste and some cosmetics to give them antibacterial properties. The FDA has recently stated that there is little evidence that either triclosan or triclocarban infused soaps are more efficient are removing germs than soap by themselves. In response to the dubious benefit of antibacterial soaps, the FDA has actually instituted a ban on hand soaps that contain antibacterial ingredients, citing that there arent enough benefits to merit the inclusion of the substances.