Solubility Trends In Group 17 Elements
The affinity of an elements atoms for one another can be quantified using entropy as a unit. For example, it takes less energy to bring gaseous H2S together than gaseous HCl since their molecules are more similar in size.
In contrast, it takes more energy to combine FCl since fluorine has a much smaller electron-cloud diameter than chlorine . By extension, mercury will take even less energy to form alloys with gold or platinum than with iron or nickel because its larger electron-cloud diameter better accommodates them.
Which Ion Is Trigonal Planar Trigonal Planar
). In organic chemistry, planar, three-connected carbon centers that are trigonal planar are often described as having sp2 hybridization. Nitrogen inversion is the distortion of pyramidal amines through a transition state that is trigonal planar.Trigonal planar molecular geometryBond angle120° 04 hàng khác
Find The Number Of Lone Pairs And Bonded Pair Electrons In The Cl2 Lewis Structure
The lone pair is unshared electrons that not involve in any chemical bonding whereas bonded pair electrons just opposite to it, they have shared electrons that involve in chemical bonding.
As shown in the figure, the Cl2 lewis structure has 1 bonded pair electron in between two chlorine atoms because they are shareable for making the chemical bond.
And 3 lone pairs on each chlorine atom because they are unshared electrons that are not shareable for any type of chemical bonding.
Note: Only a lone pair on the central atom of the molecule considers for finding the geometry of any molecule.
In Cl2, there is no central atom, so the lone pair considered zero for determining its geometry.
Don’t Miss: What Math Do 9th Graders Take
Clo3 Molecular Geometry And Shape
Chlorine forms the central atom in the ClO3ion with oxygen atoms surrounding it. According to the VSEPR theory , the lone pair on Chlorine and the electron clouds on the surrounding oxygen atoms repel each other.
Since we know that the steric number of ClO3is 4 , we can determine that the Molecular geometry of ClO3 is Trigonal Pyramidal.
From the Lewis structure above, we know that its electronic shape is tetrahedral.
CONCLUDING REMARKS
Lets quickly summarize the salient features of ClO3
- ClO3 consists of one Chlorine atom and three Oxygen atoms
- In its most stable state, Chlorine forms three covalent bonds with the surrounding Chlorine atoms making for three bonded pairs in the center with a lone pair of Chlorine.
- ClO3 has an sp3 hybridization state.
- ClO3 has a trigonal pyramidal structure with bond angles of 109.5°.
About Priyanka
To read, write and know something new every day is the only way I see my day! Well, that rhymed. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. Having an MSc degree helps me explain these concepts better. I write all the blogs after thorough research, analysis and review of the topics. And if not writing you will find me reading a book in some cosy cafe! View all posts by Priyanka
What Type Of Bond Is Present In Clo2
As you can see, on the central atom there are 2 sigma bonds and 1 pair of electrons. So it is sp2 hybridized . ( 2 câu tr li · Câu tr li hàng u: Chlorine dioxide is an odd-electron molecule. These are somewhat rare and typically unstable What type of bond will chlorine form with oxygen?3 câu tr li2 thg 3, 2017What is the hybrization of Cl in ClO2?2 câu tr li31 thg 12, 2015Which Cl-O bond is longer between ClO2 and 2 câu tr li4 thg 7, 2018Which one has a greater bond angle, Cl2O or ClO2?3 câu tr li16 thg 9, 2017Cac kt qu khac t www.quora.com
Don’t Miss: How To Do Conversions In Chemistry
What Is The Molecular Geometry Of H2s
The electronic configuration of hydrogen atoms in H2S is
Looking at valence bond theory, we know that a single H atom forms two bonds with each S atom to make a molecule. Each bond contains two electrons, so there are four bonding electrons between H and S atoms. The two lone pairs on each S atom overlap with other teams to form six shared electron pair bonds around a central ionic core or molecule consisting of four ions since S has a valence number +4 one for each pair 2-electron bonds between it and its neighbours.
What Is The Molecular Geometry Of Brf5
Hydrogen sulphide has a linear molecular geometry, meaning that its molecular bonds run along one straight line. This molecule also has two unpaired electrons. Chlorine trifluoride has a linear molecular geometry with three unpaired electrons in its outer shll.
Brmine pentafluoride has a linear structure with five unpaired electrons on its outer surface. Chloroethane and hydrocyanic acid have trigonal pyramidal structures these molecules have three bonds running between each pair of atoms, creating an overall pyramid shape. Two different atoms are bonded at each of two opposite corners.
Read Also: What Is Gis In Geography
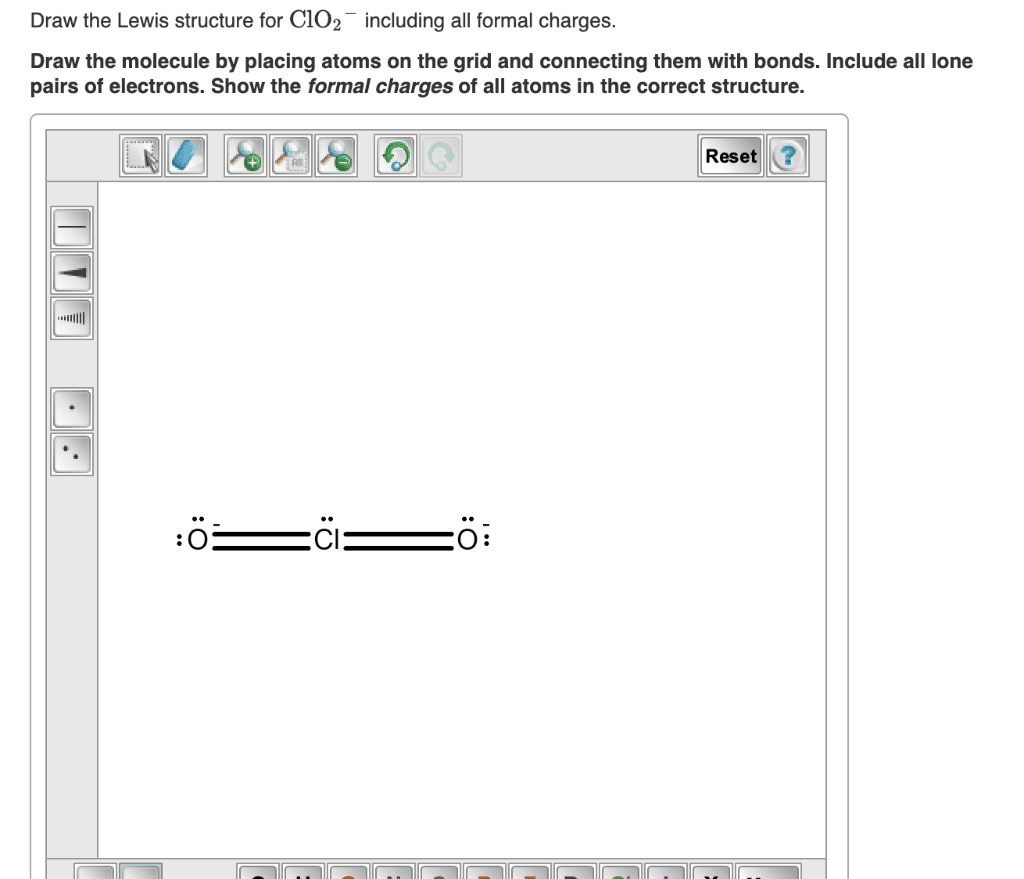
Lewis Structure Of Chlorine Dioxide
The Lewis structure is a pictorial representation of valence electrons taking part in the formation of bonds to produce a new molecule with new properties altogether.
To begin drawing the Lewis structure of Chlorine dioxide, first, it is essential to draw one for the participating elements.
For Chlorine,
Electronic Configuration = 1s2 2s2 2p6 3s2 3p5
Valence Electrons = 7
Electronic Configuration = 1s2 2s2 2p4
Valence Electrons = 6
Now, it will be easier to draw the Lewis structure of chlorine dioxide as we know that there are 20 valence electrons in one chlorine dioxide molecule.
It might confuse many people as ClO2 comprises 19 valence electrons only.
Here, it is crucial to understand that chlorine dioxide is a strong anion and oxidizing agent.
This arises from the fact that chlorine dioxide is an unstable molecule and mainly exists as ClO2- during bond formation. Due to this, the valence electrons in chlorine dioxide or chlorite are 20.
We will draw the Lewis structure of chlorine dioxide with 20 valence electrons.
Let us follow some steps to draw the Lewis structure of chlorine dioxide:
Step 1: Find the total valence electrons in one molecule of chlorine dioxide.
It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons.
There are two oxygen molecules in chlorine dioxide so the total is 19.
But chlorine dioxide exists as ClO2- during the formation of a bond, therefore we have one more valence electron available.
For Chlorine,
For Oxygen,
Polarity In Chlorine Dioxide
Chlorine dioxide is a polar molecule because it is a strong anion and exists in the ionic form.
It is important to understand that the rule of electronegativity difference will not be applicable in this case.
Although the electronegativity difference between oxygen and chlorine atoms is less than 0.4, chlorine dioxide is a polar molecule which is due to the fact that it is a strong anion.
Let us also check out its properties and uses.
Also Check: Unit 2 Formative Assessment Common Core Algebra 2
Use Vsepr Chart To Determine The Molecular Geometry Of Cl2
According to Valence shell electron pair repulsion theory if the given molecule has no lone pair on the central atom and has only one bonded pair of electrons, then the molecular geometry of that molecule will be linear and electron geometry will also linear.
In the case of Cl2, there is no central atom, so no lone pair on it, and Cl2 has only one bonded pair of electrons that is between two chlorine atoms.
So, the molecular geometry of Cl2 is linear.
Hybridization In Chlorine Dioxide
From the AXN method, it is clear that the hybridization of chlorine is sp3.
Here it is important to understand that in the case of one more Lewis structure where chlorine and both oxygen atoms are forming double bonds, the hybridization of the chlorine atom will be sp2.
Such a Lewis structure is not a usual case because chlorine dioxide is present in ClO2- form.
In the case of the chlorite ion, mixing and inter-mixing of one 2s and three 2p orbitals takes place to form four new hybrid orbitals of similar energy.
For the sigma bond, head-on overlapping takes place whereas, for the pi bond, lateral overlapping takes place.
Sigma bond is more stable than the pi bond because it undergoes head-on overlapping which is much stronger than lateral overlapping of pi bond.
Read Also: Who Are Paris Jackson’s Biological Parents
Why The Molecular Geometry Of Co2 Is Linear
The molecular geometry of CO2 is linear. Because the carbon central atom has no lone pair and is attached to the two oxygen atoms. So, there are two regions of electron density around the carbon central atom, based on VSEPR theory, it will acquire linear molecular geometry.
A region of electron density means the group of bonding or nonbonding electrons that present around the atom.
The single bond, double bond, or even triple bond around the atom will be counted as one region.
The electron pair around the carbon central atom will repel each other and tried to go far from each other, they will take the position where repulsion becomes minimum between them.
According to the VSEPR theory, the central atom with two regions of electron density adopts a linear molecular geometry because repulsion is minimum in electron pairs at this position.
Hence, the molecular geometry or shape of CO2 appears linear.
Co2 Lewis Structure Hybridization Molecular Geometry And Mo Diagram
Carbon dioxide is a colorless gas that is well known to many of us!
From the time we were in school, we knew that we inhale oxygen and exhale CO2. But is that all we need to know?
Probably no! As we indulge more in chemistry, we can see, there are many more things related to CO2. To learn all those things smoothly we need to know about this gas in more detail.
So without further adieu, lets quickly jump into the Carbon dioxide world!!
This gas has a different use in different fields. From being used as a refrigerant to carbonated beverages, CO2 is everywhere. We must not forget how important it is for the plants!!
It is used in different industries as well.
The molar mass of CO2 is 44.009 g/mol and density is 1562 Kg/m3. Now let us learn the basic concepts about CO2 molecules.
You May Like: Did Michael Jackson Have Any Biological Children
What Is The Molecular Geometry Of Clo2
The molecular geometry of ClO2 is a bent or V-shape, according to Bristol ChemLabS. ClO2 is the molecular formula for chlorine dioxide. It is a yellowish-green gas that crystallizes to bright orange crystals at -59 degrees Celsius. ClO2 is a potent oxidizing agent used for bleaching and for water treatment.
Chlorine dioxide has an odd number of valence electrons and is considered a paramagnetic radical. The compound is composed of a central chlorine atom with two oxygen atoms connected via covalent bonds. Chlorine dioxide has two resonance structures with a double bond on one side and a single bond and three electrons on the other. Both resonance structures have a bent molecular geometry with an O=Cl-O bond angle of 117.6 degrees. Chlorine dioxide is prepared in a laboratory by the oxidation of sodium chlorite, or NaClO2. CL2 is used as the oxidizing agent, acting on NaClO2 to produce chlorine dioxide and NaCl. Chlorine dioxide is used for the bleaching of wood pulp as an alternative to pure chlorine. Using chlorine dioxide minimizes the amount of toxic organochlorine compounds that are produced as byproducts. For water treatment, chlorine dioxide is less corrosive than chlorine and is more effective at killing certain waterborne pathogenic microbes such as viruses, bacteria and protozoa.
What Are The Electron And Molecular Geometry Of Cl2
The molecular geometry of Cl2 is linear as all diatomic molecules in chemistry have linear geometry because diatomic molecules contain two atoms that are connected with a single bond that produces only a straight line geometry. So, any other geometry is not possible for this type of molecule.
Linear geometry is the simplest arrangement of atoms in a straight line. The electron geometry for Cl2 is also linear. Just remember all diatomic molecule or any molecule that have only two atoms, there geometry or shape will be linear. Thats it.
Some easy steps to determine the geometry of any molecule.
Here, we need to find the molecular geometry of Cl2. So, follow these given steps to determine its shape or geometry.
As we have already drawn the lewis structure of Cl2, so directly start with step 2.
You May Like: What Is Geography In Simple Words
Stability Of The Periodic Table Trend In Group 17 Elements
One trend in Group 17 elements that stand out among all others is that as you go down a group to a minor feature, regardless of whether it is a halogen or not. This trend can be seen when looking at radii or first ionization energy.
This could be due to an inert pair effect making these elements more stable than those with no idle pairs . With that being said, two exceptions are ICl which has a larger ionic radius than IBr and ICl, which has lower first ionization energy than Br or Cl. These two exceptions can be explained by reasons different from an inert pair effect.
Molecular Geometry & Shape Of Clo2
Lewis structure has a lot of drawbacks even though it is one of the foremost and simplest methods to discuss chemical bonding. It is a 2- dimensional model and does not reflect the molecular design, geometry, or 3- dimensional representation of atoms.
ClO2 has bent molecular geometry. Therefore, chlorine is at the center, and two oxygen surrounds it.
We can also determine ClO2 molecular geometry and shape according to VSEPR theory. For that, we have to use the AXnEx method.
AXnEx notation for acetonitrile molecule:
A is the representation for central atom, so in ClO2, Chlorine atom bonded with two oxygen atoms which mean A = Chlorine
X represents the number of bonded atoms with the central atom, i.e., chlorine atoms bonded to two oxygen atoms and hence n stands for 2.
N represents the total number of lone pairs of electrons present in the central atom. There is two lone pair of electrons present on the central chlorine atom and hence x stands for 2.
Now the generic AXnEx formula for ClO2 molecule will be AX2E2 or AX2E2.
According to VSEPR theory, if the molecule has a generic formula AX2E2 then its molecular geometry will be bent and electron geometry will be tetrahedral.
Don’t Miss: How Does Physics Relate To Chemistry
Molecular Geometry & Shape Of Clo3
Lewis structure has a lot of drawbacks even though it is one of the foremost and simplest method to discuss chemical bonding. It is a 2- dimensional model and does not reflect the molecular design, geometry, or the 3- dimensional representation of atoms.
Now, Valence Shell Electron Pair Repulsion theory suggests an AXE notation.
In AXnEx notation,
A stands for the central atom, here chlorine acts as the central atom.
X represents for the number of surrounding atoms, here three oxygen atoms associated with the center chlorine atom, and hence n stands for 3.
E stands for the number of lone pairs attached to the central atoms, here one lone pairs present on the central chlorine atom, and hence x stands for 1.
Therefore, the ClO3 molecule has AX3E notation. According to VSEPR theory, if the molecule has a generic formula AX2E3 then its molecular geometry will be trigonal pyramidal but electronic geometry will be tetrahedral.
Clo4 Lewis Structure Molecular Geometry Hybridization And Polarity
Perchlorate ion or – is one of the most abundantly found ions in salts. Do you know that you can find the presence of ClO4 even on Mars?
From food packaging to acting as oxidizers in propellants for rockets, it has quite a varied range of useful properties.
However, its exposure to drinking water and edible vegetables can be harmful to the human body and can cause severe unwanted effects.
Perchlorate ion has a molar mass of 99.45 g/mol and usually forms salts with the likes of sodium, ammonium, potassium. It is usually in colorless solid form although it can also form acid .
Let us now have a quick and detailed look into the nature of the chemical bonding of ClO4.
You May Like: What Is An Independent Variable In Math
Molecular Geometry Of Clo3
After learning about the hybridization and Lewis structure we can move to the molecular geometry of this compound.
But to properly understand its structure we have to know about the theory which helps us in deciphering the shape of any compound.
This theory is the VSEPR theory.
Lewiss structure tells us about the shape of the compound but only in a 2D perspective.If you want to know about how the compound is going to appear in 3D or a planar representation then we need VSEPR theory.
With the help of the VSEPR theory, we can also find the bond angles and bond length of compounds. Now let us find out the shape of ClO3-, the compound we are learning about.
To find the right shape we will need to take a look at the VSEPR model.
The general formula used here has A, X, and E representation.
Let us put our compound in this formula.
Here, A is Chlorine, X is Oxygen and E is the number of lone pairs on the central atom which in our case is Chlorine.
If we put the values correctly then we see the formula to come at AX3E. See the diagram above and find the shape corresponding to AX3E. It is Trigonal Pyramid.
We can also find the bond angle with the help of this deduction.
The bond angle between oxygen and chlorine atom is somewhere around 109 degrees. This angle is made due to the repulsion between lone pair of electrons and bonded pairs.
Now, as we have learned about the molecular shape of the compound let us move ahead and see what is the polarity of ClO3-.