Applications Of Surface Tension
Surface tension has a huge role in daily life, health and many industrial processes. There are so many techniques that have been developed to modify surface tension.
1. Daily life Example:
a) Small insects such as the water strider can walk on the surface of the water because their weight is very less so they cant penetrate the water.
b) Disinfectants are mainly the solution of low surface tension so that when we use them in the field they can float on the water and spread out on the cells to destroy them.
c) Soaps and detergents also work on the basis of surface tension. They lower the surface tension of the water so that the soaps and detergents easily soak into the pores and holes.
d) The water bubbles are round because the surface tension of water provides the tension to form the bubble with the water and the surface tension minimizes the bubble into spherical shapes.
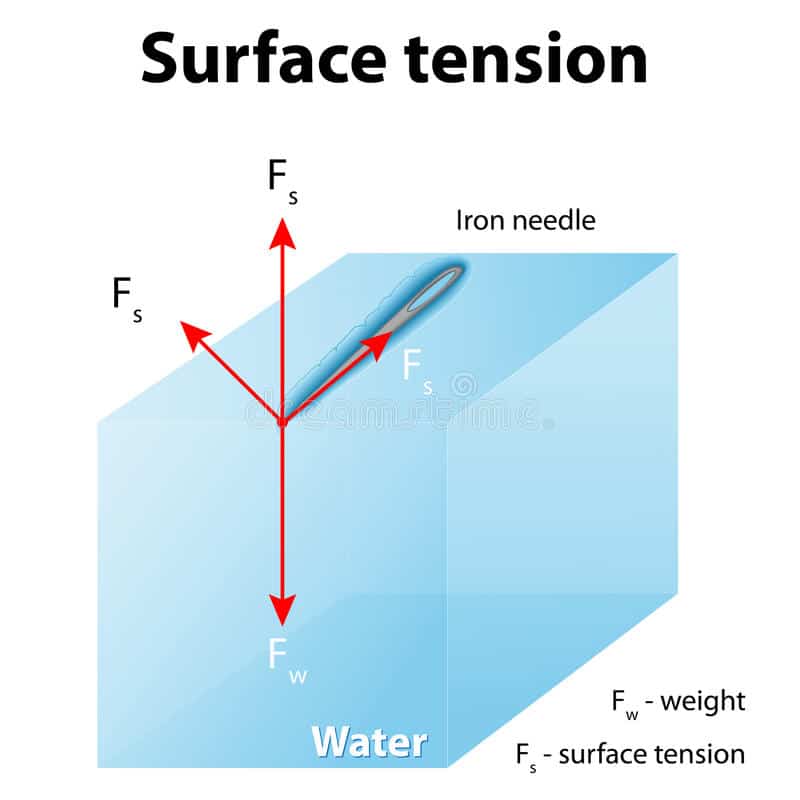
e) A small needle can be floated on the surface of the water.
2. Role of surface tension on human health:
Surface tension changes in biological phenomena can determine various diseases in the human body.
3. Industrial applications:
4. Surface tension is also important for characterization for food, pharmaceutical and packaging products.
Solution For Problem 7q Chapter 12
Introductory Chemistry | 5th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Introductory Chemistry | 5th Edition
What is surface tension? How does it depend on intermolecular forces?
Pressure Inside A Soap Bubble
To consider the pressure inside the soap bubble, we consider the radius R of the bubble and also the surface tension, gamma, of the liquid .
We begin by assuming no external pressure . You then consider a cross-section through the center of the bubble.
Along this cross section, ignoring the very slight difference in inner and outer radius, we know the circumference will be 2piR. Each inner and outer surface will have a pressure of gamma along the entire length, so the total. The total force from the surface tension is, therefore, 2gamma .
Inside the bubble, however, we have a pressure p which is acting over the entire cross-section pi R2, resulting in a total force of p.
Since the bubble is stable, the sum of these forces must be zero so we get:
2 gamma =porp= 4 gamma / R
Obviously, this was a simplified analysis where the pressure outside the bubble was 0, but this is easily expanded to obtain the difference between the interior pressure p and the exterior pressure pe:
p – pe= 4 gamma / R
Recommended Reading: Why Is Math So Hard For Me
Surface Tension Questions With Solutions
Q1. Surface tension may be defined as-
The work done per unit area in increasing the surface area of a liquid under isothermal conditions.
The work done per unit area in increasing the surface area of a liquid under adiabatic conditions.
The work done per unit area in increasing the surface area of a liquid under both isothermal and adiabatic conditions.
Free surface energy per unit volume.
Correct Answer
Q2. Water does not spread over the surface of oil because-
the surface tension of water is very high.
the surface tension of water is very low.
the viscosity of oil is high.
the viscosity of water is high.
Correct Answer- the surface tension of water is very high.
Explanation. Water has a higher surface tension than oil. As a result, when oil is poured over water, the higher surface tension of the water pulls the oil in all directions, causing it to spread on the water. Water, on the other hand, does not spread over oil because the surface tension of oil is lower than that of water, making it unable to pull water over it.
Q3. Small droplets of a liquid are usually more spherical in shape than larger drops of the same liquid because-
Force of surface tension is equal and opposite to the force of gravity.
Force of surface tension predominates the force of gravity.
Force of gravity predominates the force of surface tension.
Force of gravity and force of surface tension act in the same direction and are equal.
Correct Answer-
Real fluids.
Both ideal and real fluids.
W = mg
Quarters In A Full Glass Of Water
Needed materials:
- 10 to 12 Quarters
- glass full of water
Slowly, and with a steady hand, bring the quarters one at a time to the center of the glass. Place the narrow edge of the quarter in the water and let go.
As you continue with more quarters, you will be astonished how convex the water becomes on top of the glass without overflowing!
Possible Variant: Perform this experiment with identical glasses, but use different types of coins in each glass. Use the results of how many can go in to determine a ratio of the volumes of different coins.
Read Also: Geometry Chapter 3 Assessment Book
Which Substances Increase Surface Tension
The surface tension of water, for example, will increase when highly soluble impurities are added to it. When detergent is added to water, it decreases the surface tension of the water. Compounds that lower waters surface tension are called surfactants, which work by separating the water molecules from one another.
Surface Tension At A Molecular Level
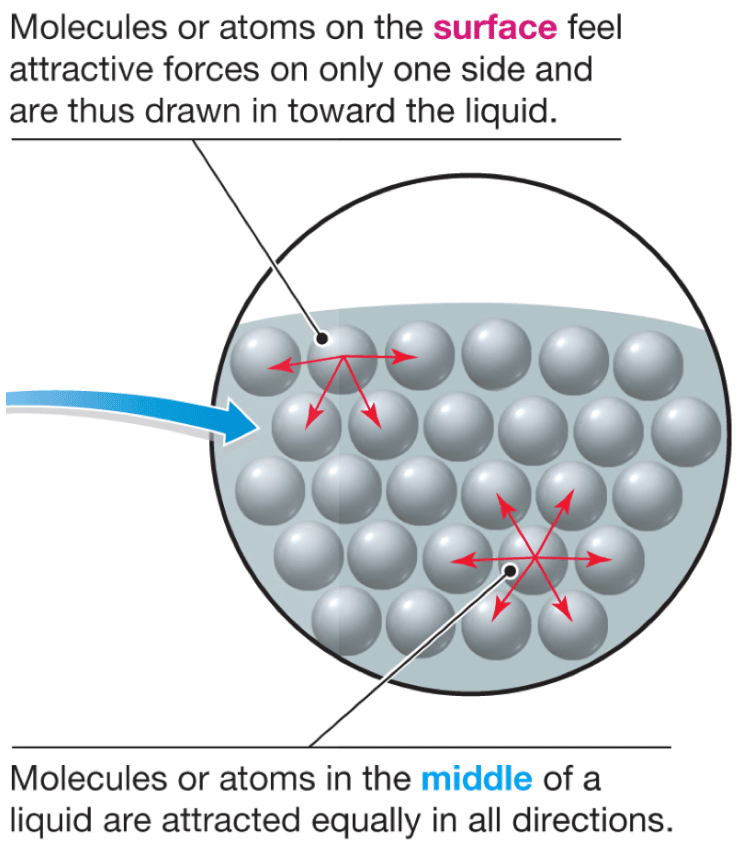
Water molecules want to cling to each other. At the surface, however, there are fewer water molecules to cling to since there is air above . This results in a stronger bond between those molecules that actually do come in contact with one another, and a layer of strongly bonded water . This surface layer creates a considerable barrier between the atmosphere and the water. In fact, other than mercury, water has the greatest surface tension of any liquid.
Within a body of a liquid, a molecule will not experience a net force because the forces by the neighboring molecules all cancel out . However for a molecule on the surface of the liquid, there will be a net inward force since there will be no attractive force acting from above. This inward net force causes the molecules on the surface to contract and to resist being stretched or broken. Thus the surface is under tension, which is probably where the name “surface tension” came from. .
Due to the surface tension, small objects will “float” on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. When an object is on the surface of the fluid, the surface under tension will behave like an elastic membrane.
You May Like: What Is Evolution In Biology
Faqs About Surface Tension
Q.1. Is there any difference in surface tension values among tap water, distilled water?
Answer: The surface tension value depends on the number of impurities there are in the water. So if we observe clearly there are more impurities in the tap water than the distilled water.
Q.2. Why rain waters form beads on the surface of the leaf?
Answer: As the surface of the leaf is waxy so the water adheres weakly to wax and strongly to itself hence they forms clusters into drops. The surface tension water gives them spherical shapes.
Q.3. How water striders walk on the water?
Answer: Objects that are less denser than that of water can float on the surface of the water. The water striders use the surface tension of the water to walk on the surface of the water.
Q.4. What are the units of surface tension?
The units of surface tension are Newton per metre and Dyn/cm .
Q.5. What are the methods of measurement of surface tension?
Answer: We can measure the surface tension by using these methods-
Q.6. Why raindrops are spherical?
Answer: The raindrops are spherical because of the cohesive forces between the rainwater molecules and the surface tension of the water molecules.
Q.7. Which are the main forces act to create surface tension?
Answer: The main forces are the cohesive force and adhesive force.
Q.8. What is the surface tension of water at boiling point?
Answer: The surface tension of water is zero at boiling temperature.
How Surface Tension Works
At the interface between a liquid and the atmosphere , the liquid molecules are more attracted to each other than they are to the air molecules. In other words, the force of cohesion is greater than the force of adhesion. Because they two forces are not in balance, the surface may be considered to be under tension, like if it was enclosed by an elastic membrane (hence the term “surface tension”. The net effect of cohesion versus adhesion is that there is an inward force at the surface layer. This is because the top layer of a molecule is not surrounded by liquid on all sides.
Water has an especially high surface tension because water molecules are attracted to each other by their polarity and able to engage in hydrogen bonding.
Read Also: What Are Isomers In Biology
Materials For Each Group
Project the image Why Water Beads.
Explain to students that waters surface tension is based on the attractions between water molecules at the surface and the water molecules in the rest of the water. A water molecule beneath the surface feels attractions from all the molecules around it. But the molecules at the surface only feel attractions from the molecules next to them and beneath them. These surface molecules are pulled together and inward by these attractions. This inward pull has the effect of compressing the surface molecules which form a tight arrangement over the waters surface. This tight arrangement at the surface is called surface tension.
The inward pull from the attractions of the molecules results in the smallest possible surface for a volume of water, which is a sphere. This is why water forms a round drop or dome at the top of the filled test tube and on the surface of a penny.
Explore
Viscosity And Surface Tension
Viscosity and surface tension, are dependent on molecular interactions.
- Viscosity results due to collaboration among molecules of identical molecules located in the same material .
- Whereas surface tension is determined by the difference in interactions between the molecules of the material with the molecules of the material in contact.
Don’t Miss: Why Physics Is Important In Engineering
What Is Surface Tension Give Two Examples
Examples of Surface Tension Insects walking on water. Floating a needle on the surface of the water. Rainproof tent materials where the surface tension of water will bridge the pores in the tent material.
What is surface tension GCSE?
Surface tension is a force between molecules in the surface of a liquid. It occurs because the molecules in the surface of the liquid attract each other, so if the are forced apart slightly as below by the weight of the pond.
Water And Alcohol On Pennies
Explain that waters attraction pulls itself together into a tight arrangement. Explain that alcohol molecules dont have a structure that is as good as waters for attraction to itself. Alcohol molecules only have 1 OH bond and they have some CH bonds that are pretty non polar. There is not as strong an attraction between them as there is between water molecules.
The shape of the water molecule and its polarity at the top and the bottom give water molecules lots of opportunities to attract. Almost anywhere two water molecules meet they can be attracted to each other.
But alcohol has a different size and shape and has its polar part on one end. Alcohol molecules can meet at areas where they would not attract as strongly. The water is more attracted to itself than to the metal of the penny. The alcohol is a bit less attracted to itself so it spreads more on the penny.
Explore
Recommended Reading: Is Quantum Mechanics The Same As Quantum Physics
Discuss How The Polarity Of The Material That The Water Is Placed On Affects How The Water Absorbs Or Beads Up
Ask students:
- If water absorbs into a paper towel but does not absorb into wax paper, what does that say about the polarity of paper and wax paper?
- The molecules that make up paper are probably polar, and the molecules that make up wax are probably nonpolar.
Explain that paper towel and other paper is made from cellulose. Cellulose is made from repeating molecules of glucose that are bonded together. The glucose molecule has many OH bonds, which are polar. Polar water molecules are attracted to polar cellulose.
Tell students that wax is made from paraffin, which is repeating carbon-hydrogen bonds. The CH bond is not very polar, so water is more attracted to itself than to the wax. This causes the water to bead up on wax paper.
Define The Following Terms And Describe How Each Depends On The Strength Of The Intermolecular Forces A Surface Tension D Boiling Point B Viscosity E Vapor Pressure C Melting Point
a.Surface tension:
Surface tension is known as the tendency of any liquid surface at rest to shrink into the minimum surface area possible. Surface tension is a unique property of liquids that depends on the nature of intermolecular interactions between the molecules of liquids. An increase in the intermolecular interactions between the molecules causes the surface tension to increase.
b.Viscosity:
The viscosity of a fluid is used to measure the resistance to deformation of fluid at a given rate.
Intermolecular forces are essential in defining viscosity because molecules have strong attractions between them. It causes them to resist flow more strongly.
c.Melting point
The melting point of a substance is the temperature at which substances change state from solid to liquid. When a strong intermolecular force acts between the molecules of substances, more energy is needed to melt the molecules. So the melting point is higher.
d.Boiling point
The boiling point of a substance is the temperature at which the vapor pressure is equal to the standard sea-level atmospheric pressure. Compounds with strong intermolecular forces between their molecules will have higher boiling points.
e. Vapor pressure
Don’t Miss: What Is Migration In Geography
What Effect Does A Surfactant Have On Surface Tension
The cohesive forces between the water molecules are very strong making the surface tension of water high. As surfactants absorb they break these interactions. The intermolecular forces between surfactant and water molecule are much lower than between two water molecules and thus surface tension will decrease.
What increases surface tension water?
The Presence of ImpuritiesThe presence of impurities on the surface of, or dissolved in, a substance directly affects the surface tension of the liquid. The surface tension of water, for example, will increase when highly soluble impurities are added to it.
When Does Surface Tension Occur Between Two Liquids
Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with gas, acts like a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas . If the surface is between two liquids , it is called interface tension.
Whats the difference between surface tension and surface energy?
Surface tension. The two are equivalentbut when referring to energy per unit of area, people use the term surface energy which is a more general term in the sense that it applies also to solids and not just liquids. In materials science, surface tension is used for either surface stress or surface free energy .
Read Also: What Is Grid In Geography
What Causes Surface Tension
Surface tension has been well- explained by the molecular theory of matter, and Laplace explained this phenomenon based on intermolecular forces. For example, we know that if the distance between two molecules is less than the molecular range \\\), they attract each other. Still, if the distance is more than this, they attract each other, but the attraction force decreases considerably with distance.
Therefore, if we draw a sphere of radius \ with a molecule at its centre, only those enclosed within this sphere can attract, or be attracted by, the molecule placed at the spheres centre. This is called the sphere of molecular activity.
To understand the tension acting in a liquids free surface, let us consider four liquid molecules like \ and \ along with their spheres of molecular activity. The molecule \ is well inside the liquid, and so it is attracted equally in all directions. Hence the resultant force acting on it is zero. The sphere of molecule \ is just below the liquid surface, and the resultant force on it is also zero.
When the surface area of liquid is increased, molecules from the interior of the liquid rise to the surface as these molecules reach near the surface, work is done against the cohesive force. This work is stored in the molecule in the form of potential energy.