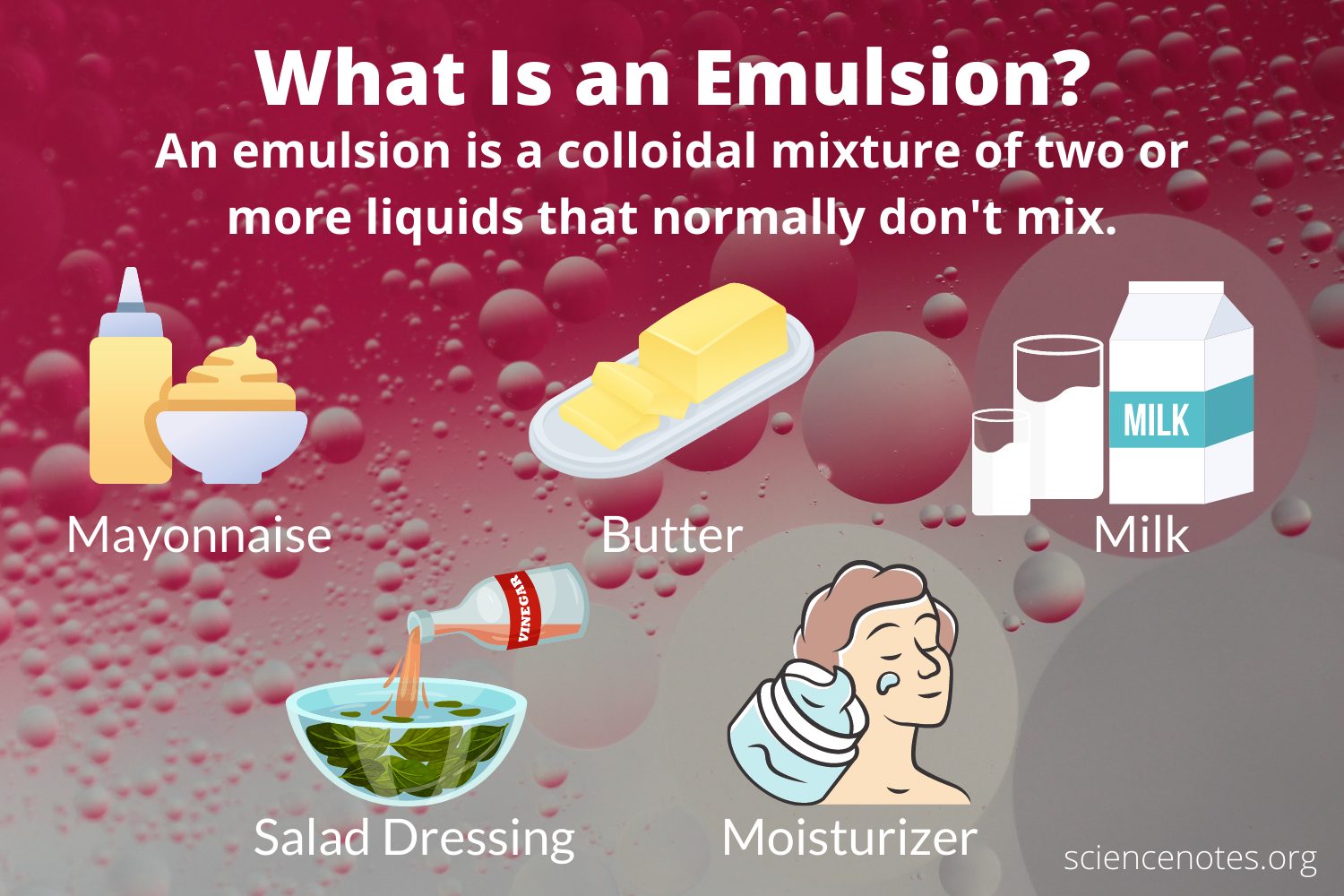
Applications And Uses Of Emulsion
Emulsions are very much famous in various fields of science. It is utilized in the tanning and dyeing industries, used in the manufacturing process of plastics and synthetic rubber.
- Usually used in cosmetics, pharmaceuticals, personal hygiene.
- Microemulsions are used to deliver vaccines to kill various microbes.
- It is used in chemical synthesis mainly in the manufacture of polymer dispersions.
- It is used in firefighting.
- Nanoemulsions such as soybean oil are used to kill microbes.
- Mayonnaise is an oil in water emulsion with egg yolk or sodium stearoyl lactylate.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Deeper Level Chemistry For Secondary Pupils
Water is a polar molecule meaning it has positively and negatively charged ends. This polarity is created because the electrons are attracted to the oxygen atom of the molecule more than the hydrogen atom . The density of electrons makes the oxygen slightly negative while the hydrogen will be slightly positive .
The polarity means that water molecules can attract each other. When salt is put into water, it breaks down to form charged ions: a positively charged sodium ion and a negatively charge chloride ion . The water is attracted to the ions and surrounds them so the ions are mixed evenly throughout the water. We call this dissolving. So ions or molecules with a slight or strong charge can dissolve in water.
Oil does not have a polarity so it is not attracted to the water. Oil molecules will clump together. They are known as hydrophobic molecules from the Greek hydro, meaning water phobos, meaning fear. Sugar does dissolve in water because it has a slight negative charge that is attracted to the water. Sugar is known as a hydrophilic molecule from the Greek hydro meaning water philia, meaning love.
Characteristics Of The Emulsion
Emulsion is a term that people rarely hear, but despite this it has great importance in many areas such as cooking and medicine . Some of its most outstanding characteristics are:
- This process is used by chefs and foodies , but above all by Italian families, who like to cook different sauces and creamy dishes. Although it is also essential to use this tool to create things like cream or butter .
- Within medicine, it is widely used in various vaccines that use fatty products such as soybean oil to create anti-pathogens.
- Within the area of care, many companies use these methods to make their facial creams that have a more or less liquid consistency.
- The emulsion is also found within photography and refers to the suspension of a silver bromide in gelatin, it refers to one of the photographic material which is sensitive to excess light.
Also Check: Which Founding Contributors To Psychology Helped Develop Behaviorism
What Is Emulsion In Chemistry
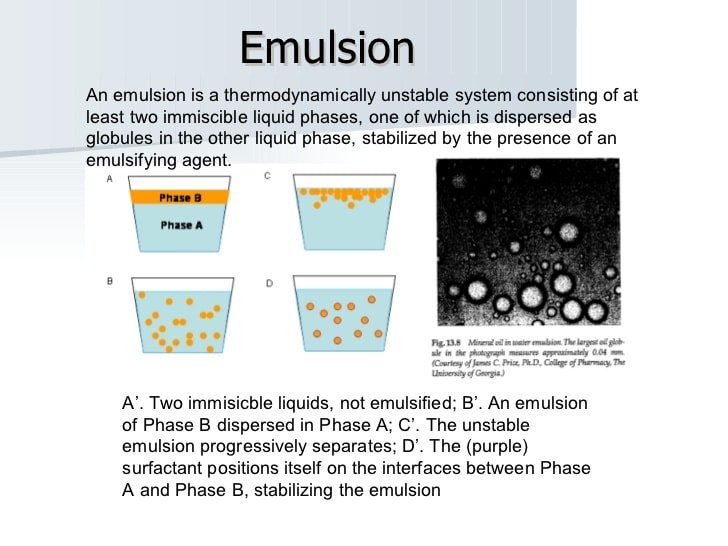
In physical chemistry, emulsion is a combination of two or more liquids, one of which is present as droplets, microscopic or ultramicroscopic in dimension, scattered over the other. Eventually, turbulent emulsions split into two liquid layers.
A combination of two liquids that would not necessarily be combined is an emulsion. That is to say, a combination of two liquids which are immiscible. An emulsion comprises tiny fragments of one liquid suspended in another, by definition. When mixed slowly under intense stirring, oil and water are a typical example of an emulsion.
Was this answer helpful?
What Is An Emulsifier
Emulsifiers are substances which stop liquids in an emulsion separating. Emulsifier molecules have two different ends. One end is hydrophilic and the other end hydrophobic . In the case of oil and water, the hydrophilic end forms a bond with the water and the the hydrophobic end forms a bond with the oil!
Recommended Reading: Define Abiotic Biology
Basic Sediment And Water
Basic sediment and water is the solids and aqueous portion of an emulsion. It is also referred to as BSW, bottom settlings and water, or bottom solids and water. Several methods are available to determine the amount of water and solids in emulsions. Standard methods have been proposed by several organizations including the :
- Institute of Petroleum
- American Society for Testing Materials
The most common technique for the determination of oil, water, and solids consists of:
The amount of solids and water separated is measured directly from specially designed centrifuge tubes. When only the water content is desired, Karl-Fischer titration can also be used. It is very accurate at low contents of water but can also be used for determining higher content . Other, less common methods are based on :
- Electrical properties
- Gamma-ray attenuation
- Microwave-based meters
Understanding Emulsions In Foods
by Institute of Food Technologists
An emulsion is a mixture of two fluids such as oil and water that is achieved by breaking up the molecules in both substances into very fine, small droplets in order to keep the combination from separating. In the August issue of Food Technology magazine published by the Institute of Food Technologists Contributing Editor, J. Peter Clark breaks down what emulsifiers are and how they are used in familiar foods.
There are several common foods that are considered emulsions: milk, , ice cream, mayonnaise, salad dressings, sausages, and sauces like béarnaise and hollandaise. When packaged and manufactured on a larger scale, most of these foods need emulsifiers to stabilize the mixture and keep the different ingredients from becoming separated. Lecithin is a common emulsifier that is naturally found in soy oil. Egg yolks are another example of an emulsifier they contain lecithin and cholesterol, which makes them a great binder for sauces like mayonnaise.
The way emulsifiers work is by coating the molecules of a certain fluid, making it easier to mix with the other ingredients, and also keeping the mixture together over a longer period of time without separating. In the kitchen this can be achieved by vigorous beating or whisking, or using a hand mixer. Using an emulsifier correctly in a sauce or mixture requires adding the ingredients in the proper order, and often at a specific temperature.
Read the full Food Technology article here.
Recommended Reading: Who Are Paris Jackson’s Biological Parents
How Do Amphiphilic Emulsifiers Work
Amphiphilic molecules have parts of them that prefer to sit in the water phase and parts that prefer to sit in the fatty phase . In an emulsion the molecules will arrange there hydrophibic sides into the fat and their hydrophilic sides in the water. See below for a simplified illustration.
There are a lot of these amphiphilic emulsifiers, but well discuss two main categories in more detail.
Proteins
Proteins are large complex molecules built up of a long chain of amino acids. Each amino acid has a side chain and this side chain can be hydrophilic or hydrophobic. Through all these different amino acids proteins will have sections which are hydrophobic and sections which are more hydrophilic.
In order for proteins to properly be surface active they will have to unfold partly. Some of this can occur spontaneously, but it can also be help along, for example by whipping proteins . Since these proteins are quite large and bulky, their bulkiness helps stabilize an emulsion. They form some sort of a layer around the particles which makes it harder for like particles to find each other.
Surfactants
S To Identify The Type Of Emulsions
1) Dilution test
On adding water to an o/w emulsion, it will still remain stable as water is the dispersion medium, but on adding oil it will get destabilized as oil & water are immiscible. Similarly, w/o emulsion can be diluted with oil & would still be stable, but would get destabilized on the addition of water.
2) Conductivity test
In this test the emulsion is kept between 2 electrodes and a bulb is connected in the circuit as shown in the diagram. An o/w emulsion will conduct electricity as water conducts electricity, but a w/o will not conduct electricity.
3) Dye test
In this, a water-soluble dye is added to the emulsion. If it is an o/w emulsion, the dispersion medium appears red and the dispersed phase colourless and vice-versa.
You May Like: Math Road Trip Project
What Is An Emulsion
When talking about what an emulsion is , it is nothing more than a mixture which seeks to bind in a relatively homogeneous way two liquids of different compositions. The union system seen in simpler terms is something very easy to understand the main liquid is to be completely diluted in the other. The process that contemplates the preparation of emulsions is called emulsification. In general, the emulsions that are commonly made are those of oil or water with various dietary fats that generally come from nature.In a little deeper terms, emulsions are included within physics as two-phase systems of matter called colloids .
- Definition
- Examples
What Is An Egg Emulsion
All good recipes for hollandaise sauce consist of beating egg yolks vigorously and slowly add melted butter over moderately low heat. Care must be taken to not overheat the sauce or the eggs will scramble and it won’t emulsify. Likewise, the melted butter must not be added too quickly or it will not emulsify. When done properly like a good chef, what is happening here? Why does the sauce thicken? What is an egg emulsion and why must the butter be added so slowly rather than faster?
To know what’s happening here you should know what “emulsifying” is.
First we need to know that fat is “non-polar” substance. what does that mean?
It means that all fats can’t be dissolved in water which is “polar” substance.
So how can we make fat -which is non-polar- dissolve in water or any other polar substances?
We add “emulsifiers” like: egg yolk and many other chemical substances like Lecithin.
These emulsifiers are so they can link fat with water together.
We add butter slowly so that the molecules of emulsifier can link the molecules of butter with molecules of sauce -which is polar-.
If there’s no free molecules of emulsifier, every molecule of added butter will not be dissolved in the sauce.
Read Also: Eoc Fsa Warm Ups Algebra 1 Answers
Emulsion Liquid Membrane System
An ELM is a three-phase system and can consist of water/oil/water or oil/water/oil phases. In either system, the liquid membrane is the phase separating the like phases. For a W/O/W system, the oil phase separating the two aqueous phases is the liquid membrane phase . The ELM process can be described in four steps, which are shown in Figure 1.
Figure 1. Emulsion Liquid Membrane Process. From Correia, P. F. M. M., de Carvalho, J. M. R. J. Membr. Sci.2000, 179, 175â183.
How Do Emulsifiers Work
To understand this, firstly we need to understand the process of coalescing. Coalescing is the process in which the similar particles in the emulsions come together to form larger and bulkier particles leading to the separation of the dispersed phase and dispersion medium.
Emulsifiers help in preventing coalescing by forming a physical barrier between the dispersed phase and dispersion medium. As we have seen before emulsifiers, like soap, has both a hydrophilic end and a hydrophobic end. Hence, they can attach to both polar and non-polar substances. Let us take the example of sodium stearate. C17H35COONa can be represented as
When this is added to an o/w emulsion, molecules of C17H35COO surround the oil droplet with their non-polar tails/hydrophobic end extending into the oil & their polar heads/hydrophilic end facing the water as given in the figure.
This arrangement brings a stronger adhesive force between the oil and water . This new formed adhesive force will be more than the cohesive force between oil oil and water water. Hence, oil particles will not have the tendency to come together to form larger particles. This helps in preventing coalescing, thereby stabilizing the emulsion.
Note:
For w/o emulsion, the orientation of the emulsifier would be the opposite as that of o/w. i.e. non-polar tail extends outside and polar head faces inwards.
Also Check: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
How It Differs From Suspension
Actually when the theoretical bases of what is the emulsion and what is the suspension are analyzed, it can be understood that only a small line of difference separates them. Although it is true that the emulsion is the colloidal separation of a liquid within another, the emulsion is also a colloidal separation but of a solid within a liquid, for example salt in water.
Droplet Size And Droplet
Produced oilfield emulsions generally have droplet diameters that exceed 0.1 m and may be larger than 100 m. Emulsions normally have a droplet size range that can be represented by a distribution function. Fig. 5 shows the drop-size distributions of typical petroleum emulsions. The droplet-size distribution in an emulsion depends on several factors including the:
- Interfacial tension
- Nature and amount of emulsifying agents
- Presence of solids
- Bulk properties of oil and water
Droplet-size distribution in an emulsion determines, to a certain extent, the stability of the emulsion and should be taken into consideration in the selection of optimum treatment protocols. As a rule of thumb, the smaller the average size of the dispersed water droplets, the tighter the emulsion and, therefore, the longer the residence time required in a separator, which implies larger separating plant equipment sizes. The photomicrographs in Figs. 1 through 4 show the droplet-size distribution for several emulsions.
-
Fig. 5 Droplet-size distribution of petroleum emulsions.
The droplet-size distribution for oilfield emulsions is determined by the following methods.
- Microscopy and image analysis. For example, the emulsion photomicrographs in Figs. 1 through 4 can be digitized and the number of different-sized particles measured with image analysis software.
- Physical separation including chromatographic techniques, sedimentation techniques, and field-flow fractionation.
Read Also: Benefits Of Using Manipulatives In Math
What Are Emulsifiers And What Is Their Function In Food
Ever tried to make a simple dressing of olive oil and balsamic vinegar? Noticed that its impossible to keep these two mixed without continuously stirring or adding something like mustard? Wondered how food manufacturers are able to do this?
If youve looked on labels of various dressings and sauces you might have come across the word emulsifying agent or emulsifier. This is the secret to their stable dressings, its these components that prevent their mixtures from splitting. Even though their name might sound pretty chemical, emulsifiers have been used for a long period of time and the word emulsifier is simply used to cover a range of different components that all do a similar job: keep two components mixed that dont want to be mixed.
How Emulsification Works
There are a few mechanisms that may be involved in emulsification:
- Emulsification may occur when the interfacial surface tension between two liquids is reduced. This is how surfactants work.
- An emulsifier may form a film over one phase in a mixture to form globules that repel each other, allowing them to remain evenly dispersed or suspended.
- Certain emulgents increase the viscosity of the medium, making it easier for the globules to remain suspended. Examples include the hydrocolloids acacia and tragacanth, glycerine, and the polymer carboxymethyl cellulose.
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers
What Does Emulsion Mean
An emulsion is a mixture of two liquids that dont fully combine. An emulsion may look like a single liquid, but its made up of particles of one liquid distributed throughout another liquid.
For example, if you whisk together oil and water, it forms an emulsion in which small droplets of oil are suspended in the water, but the two liquids arent fully blended together .
In technical chemistry terms, an emulsion is a colloidalsuspension in which the substances mixed together are both liquids. Both colloids and suspensions involve particles of one substance distributed in another without being dissolved.
The word emulsion is used in a variety of contexts, including pharmacology, cooking, and photography.
In cooking, emulsions are made by blending two liquids or liquid-like ingredients into a smooth consistency. Salad dressings called vinaigrettes are typically emulsions of oil and vinegar.
The word emulsion is used in a more specific way in photography to refer to a light-sensitive coating thats applied to paper or film.
The verb emulsify means to form an emulsion.
Example: To properly make an emulsion of oil and vinegar, you have to whisk very hard to separate the oil into tiny droplets, or else the two liquids will separate.
Difference Between Emulsion And Colloid
Sometimes the words emulsion and colloid are used interchangeably, but they dont mean quite the same thing. An emulsion is a type of colloid. A colloid, in turn, is a type of homogeneous mixture. All emulsions are colloids, but not all colloids are emulsions. An emulsion is a colloid in which all the phases are liquids. There are other types of colloids, defined according to their phases. For example, an aerosol is a solid dispersed in a gas , while a foam is as gas dispersed in a liquid .
Read Also: Span Definition Linear Algebra
Noteworthy Papers In Onepetro
Agrell, J., & Faucher, M. S. . Heavy Oil and Bitumen Dehydration – A Comparison Between Disc-Stack Centrifuges and Conventional Separation Technology. Society of Petroleum Engineers.
Alboudwarej, H., Muhammad, M., Shahraki, A. K., Dubey, S., Vreenegoor, L., & Saleh, J. M. . Rheology of Heavy-Oil Emulsions. Society of Petroleum Engineers.
Al-Ghamdi, A. M., Noïk, C., Dalmazzone, C. S. H., & Kokal, S. L. . Experimental Investigation of Emulsion Stability in Gas/Oil Separation Plants. Society of Petroleum Engineers.
Beetge, J. H., & Horne, B. . Chemical-Demulsifier Development Based on Critical-Electric-Field Measurements. Society of Petroleum Engineers.
Dalmazzone, C., Noik, C., & Komunjer, L. . Mechanism of Crude-Oil/Water Interface Destabilization by Silicone Demulsifiers. Society of Petroleum Engineers.
Dalmazzone, C., Noïk, C., Glénat, P., & Dang, H.-M. . Development of a Methodology for the Optimization of Dehydration of Extraheavy-Oil Emulsions. Society of Petroleum Engineers.
Fjeldly, T. A., Hansen, E. B., & Nilsen, P. J. . Novel Coalescer Technology in First-Stage Separator Enables Single-Stage Separation and Heavy-Oil Separation. Society of Petroleum Engineers.