Examples Of Ways To Measure Volume
Volume is the level at which something is heard or the amount of space a solid, liquid or gas occupies. With sound, its volume is the loudness of the sound. With a container, its volume would be its capacity, or how much it can hold. Volume is often expressed in cubic units determined by the International System of Units. Learn how to measure volume with ease.
How To Calculate Volume Using Density
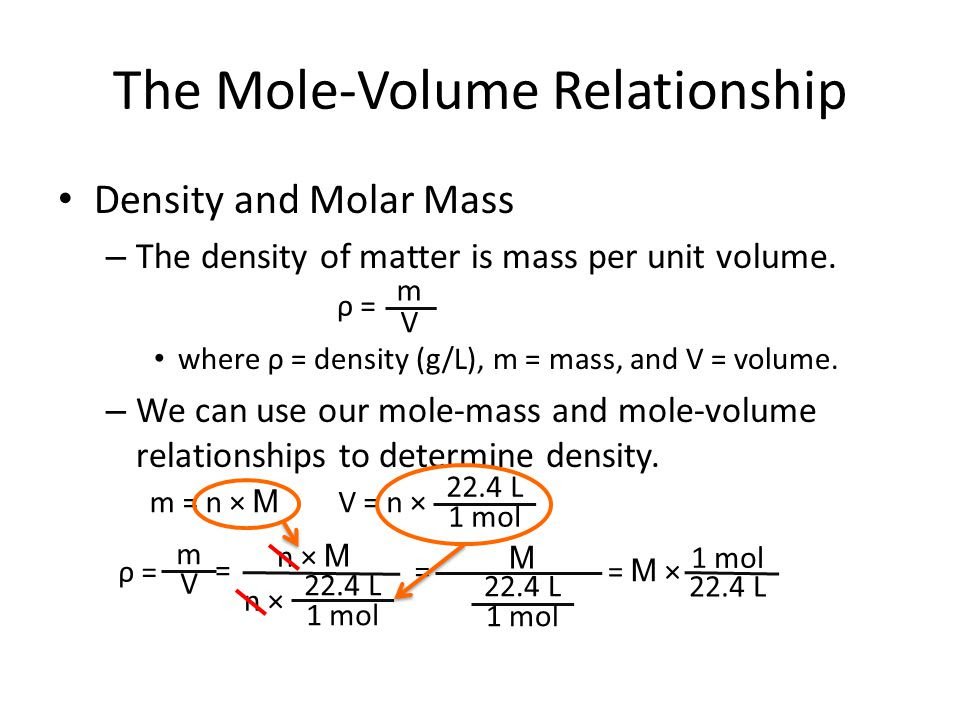
Density measure the amount of mass in a given volume of substance or how much material is in a given space. The density is constant for a substance at a given temperature since increasing the mass of a sample will increase the volume at a proportional rate. Density is calculated by dividing the mass of a substance by the volume . If the density of a substance is known, determining the mass of a sample will allow the volume to be calculated.
Determine the density of the substance. Many reference sources are available that give the density of different compounds. Commonly used references include the Merck Index and the CRC Handbook of Chemistry and Physics.
Determine the mass of the substance using a balance. Either a triple-beam balance or electronic balance may be used. One method of measuring the mass is to zero the balance with the container for the sample on the balance. Then add the sample to the container and measure the mass of the container and sample. Alternatively, the mass may be determined by measuring the mass of the container and then the mass of the container with the substance. Subtract the mass of the container from the mass of the substance and container to calculate the mass of the substance .
Related Articles
Volume And Temperature: Charless Law
If we fill a balloon with air and seal it, the balloon contains a specific amount of air at atmospheric pressure, lets say 1 atm. If we put the balloon in a refrigerator, the gas inside gets cold and the balloon shrinks . If we make the balloon very cold, it will shrink a great deal, and it expands again when it warms up.
This video shows how cooling and heating a gas causes its volume to decrease or increase, respectively.
These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: The volume increases as the temperature increases, and decreases as the temperature decreases. Volume-temperature data for a 1-mole sample of methane gas at 1 atm are listed and graphed in Figure 4.
Figure 4.
The relationship between the volume and temperature of a given amount of gas at constant pressure is known as Charless law in recognition of the French scientist and balloon flight pioneer Jacques Alexandre César Charles. Charless law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant.
Mathematically, this can be written as:
with k being a proportionality constant that depends on the amount and pressure of the gas.
For a confined, constant pressure gas sample, \frac is constant , and as seen with the PT relationship, this leads to another form of Charless law: \frac = \frac.
Also Check: Theory Of Everything 2 All Coins
Calculating The Volume Of A Cylinder
How To Measure The Volume Of Sound
The loudness of a sound can be subjective, such as quiet or loud. When it comes to science, a more objective measure of sound is used. Ways to find the volume of sound include:
- Sound pressure level – The human ear averages the SPL over a period of 600-1000 milliseconds. After about 1 second, the ear creates an average, and the level of loudness will seem to become stable.
Also Check: Holt Mcdougal Geometry Workbook Answers
Measuring The Volume Of Solids Liquids And Gases
How to find the volume of objects with different states of matter?
1. Solid
So, if you want to measure an irregular object’s volume, just follow Archimedes footsteps :
-
Take a container bigger than the object you want to measure the volume of. It may be a bucket, a measuring cup, a beaker, or a graduated cylinder. It should have a scale.
-
Pour water into the container and read the volume measurement.
-
Put the object inside. It should be totally submerged to measure the objects whole volume. Read off the volume. This method won’t work if your object dissolves in water.
-
The difference between the measurements is the volume of our object.
These measurements are essential in calculating the buoyancy force which are based on Archimedes’ principle.
2. Liquid
Usually, it’s quite easy to measure the volume of a liquid – all you need to have is a graduated measuring vessel of some kind. Choose the one that fits your needs: the amount of liquid and degree of accuracy are the parameters to consider. The containers used in baking a cake will be different to those used in chemistry will be different to ones used for medical purposes .
3. Gas
We have to use more elaborate methods to measure the volume of a gas. You need to remember that the volume of gas is influenced by temperature and pressure, and that gases expand to fill any container in which they’re placed. You can try to measure it:
Or calculate:
How Do You Find Displacement In Chemistry
Density / Water Displacement Method / Percent Error
Don’t Miss: Holt Geometry Lesson 4.5 Practice B Answers
Standard Conditions Of Temperature And Pressure
We have seen that the volume of a given quantity of gas and the number of molecules in a given volume of gas vary with changes in pressure and temperature. Chemists sometimes make comparisons against a standard temperature and pressure for reporting properties of gases: 273.15 K and 1 atm . At STP, an ideal gas has a volume of about 22.4 Lthis is referred to as the standard molar volume .
Figure 10.
Ppm By Volume Or Mass
Going through a basic PPM calculation by volume or by mass will help to cement the concepts you need to understand. For mass, imagine you have 0.98 liters of water with 0.2 g of salt dissolved in it, noting that 1 L of water = 1 kg of mass, so the total mass in the example is 1 kg. After converting both to the same mass unit, the proportion of salt in the water is given by:
So this is basically parts per part, meaning that for one part solution you have 0.0002 parts salt. But of course, having this many decimal points isnt exactly convenient, so to convert to PPM you multiply by 1,000,000 = 106, giving: 0.0002 × 106 = 220 PPM.
The general approach works for volume calculations, too. Imagine you have a sample of 1 m3 of air, with 0.0004 m3 of carbon dioxide in the mixture: What is the PPM concentration of carbon dioxide? Starting with the parts per part or parts per 1 calculation:
And then multiply this by 106 to finish the PPM conversion: 0.0004 × 106 = 400 PPM.
Don’t Miss: Sacred Geometry Moon Phases Tattoo Spine
Moles From Gas Volume
Example:Calculate the number of moles of ammonia gas, NH3, in a volume of 80 L of the gas measured at STP.
Solution:Volume of gas = number of moles × 22.414 L/mol
How to convert from liters to moles?The following video shows an example of liters to moles conversion. It shows how to convert litres of agas at STP into moles
Example:
Calculating The Volume Of A Regular Square Pyramid
Read Also: How To Find Ksp Chemistry
Volume Calculator And Tools Dedicated To Specific Shapes
We’ve decided to make this volume calculator a simple tool which covers the five most popular 3D shapes. However, not every volume equation and shape type may be implemented here, as it will make the calculator overloaded and unintuitive. So if you’re looking for a specific shape, check out the calculators dedicated to volumes of chosen shapes:
Finding The Density Of A Liquid
In many cases, you can look up the density of a liquid in a table. Some are easy to remember. For example, the density of water is 1 g/ml, which is equivalent to 1,000 kg/m3, although the value in Imperial units is a little less memorable: 62.43 lb/cu ft. Other densities, such as those of acetone, alcohol or gasoline, are readily available.
If you have a solution, you need to know the relative concentrations of solvent and solute to calculate its density. You determine this by weighing the solute before adding it to the solvent. If you don’t know the proportions, you can’t calculate density and therefore can’t derive the volume of the solution simply by weighing it.
Also Check: What Does K Stand For In Math
Finding Concentration In Percentage Or Parts Per Million
What Is The Meaning Of Water Displacement
When an object enters water, it pushes out water to make room for itself. The object pushes out a volume of water that is equal to its own volume. This is called displacement. Any object that is in water has some buoyant force pushing up against gravity, which means that any object in water loses some weight.
Also Check: Angle Addition Postulate Worksheet Answer Key
Key Concepts And Summary
The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change . The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure . The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant . Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules .
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal gas constant.
Finding The Ratio Between Mass And Volume
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Density is the measurement of the amount of mass per unit of volume. In order to calculate density, you need to know the mass and volume of the item. The formula for density is:
density = mass/volume
The mass is usually the easy part while finding volume can be tricky. Simple shaped objects are usually given in homework problems such as using a cube, brick or sphere. For a simple shape, use a formula to find volume. For irregular shapes, the easiest solution is to measure volume displaced by placing the object in a liquid.
This example problem shows the steps needed to calculate the density of an object and a liquid when given the mass and volume.
Read Also: Reasoning Minds Math Login
How To Calculate Liquid Volume
It’s usually fairly easy to calculate the volume of a liquid in a container with a regular shape, such as a cylinder or cube. All you have to do is use the appropriate mathematical equation to calculate the capacity of the container, then measure the level of the liquid and make the necessary adjustment. It’s more challenging when the container doesn’t have a regular shape, and that’s most of them. The challenge disappears if you know the density of the liquid, though. All you have to do is weigh the container and the liquid, subtract the weight of the container, and use the density of the liquid to derive your answer.
TL DR
You can calculate the volume of a liquid from its weight if you know the density of the liquid. You can usually look up density in a table. If you have a solution, you need to know the relative proportions of solute and solvent to calculate its density.
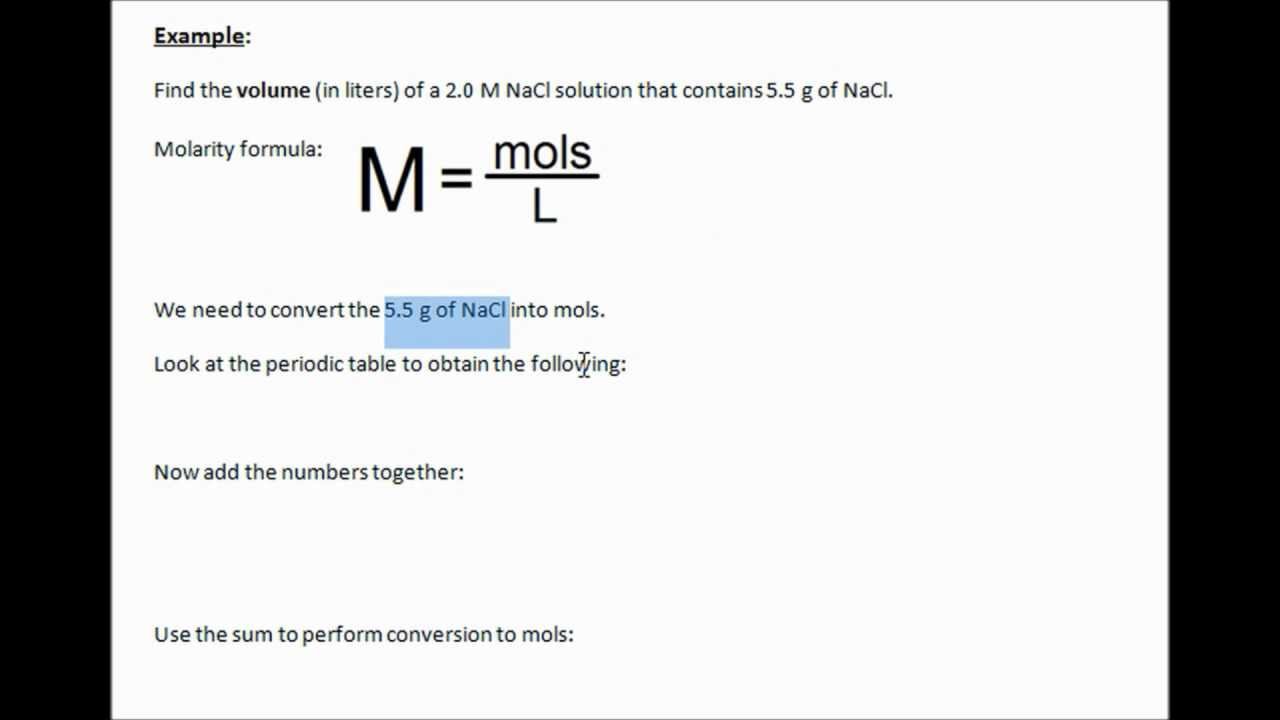
How Do You Calculate The Volume Of A Solution
4.9/5volumesolutionequationvolumesolutionvolumeto findsolution
Also asked, how do you find the volume of a solution with molarity?
Compute the volume of a solution in liters, given the number of moles and molarity, by dividing the number of moles by the molarity in units of moles per liter. For example, a solution containing 6.0 moles and a having a molarity of 3.0 moles per liter has a volume of 2.0 moles per liter.
Similarly, what is Molality formula? The formula for molality is m = moles of solute / kilograms of solvent. In problem solving involving molality, we sometimes need to use additional formulas to get to the final answer. One formula we need to be aware of is the formula for density, which is d = m / v, where d is density, m is mass and v is volume.
Keeping this in view, what is molarity of a solution?
To do this measure called molarity is commonly used. Molarity is defined as the number of moles of solute divided by the volume of the solution in liters. It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent.
What is concentration of a solution?
Concentration Definition. In chemistry, concentration refers to the amount of a substance in a defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution. Concentration is usually expressed in terms of mass per unit volume.
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers