The Truth About Isotonic Biology
Thats usually because theyre formulated with plenty of carbohydrates so as to maximise energy delivery as a means to fuel high intensity activities. Next week I aspire to learn more deeply about the purpose of different kinds of molecules going in and out of the human body. In an experiment looking for out the isotonic point of something, salt isnt the very best substance to use.
That means you will wind up with the exact same effects which you would see if you had given the patient a Hypotonic IV solution to start out with. Motion analysis using videotaping may also be useful in reinforcing correct movement patterns once biomechanical deficits are addressed. Peptidoglycan is a fantastic substance.
The Basic Facts Of Isotonic Definition Biology
Water moves freely, but doesnt will need to dilute www.papernow.org solute inside or away from the cell, hence the concentrations remain the exact same and the cells are absolutely free to do their company. Movement of water from the exterior of the cell to the interior of the cell when its put in a hypotonic environment typically results in the cell to swell. Turgidity is essential for healthier plant cells, as it assists them maintain rigidness.
There may be quite a few possible sources of random errors and their source is contingent on the sort of experiment and the sorts of measuring instruments used. Below you can discover links to futhrer examples of various varieties of solutions having a more comprehensive explanation. Some cells cant perform certain tasks in the event the ion channels arent working properly.
Lab 1D During Lab 1D, only paper, pencil, and a calculator is going to be needed to create the calculations. The machine functions as an artificial kidney and attempts to re-establish normal heights of ions and water in their bodies. It doesnt take a lot of salt to earn a considerable effect in the way in which the rinse performs and feels when you use it.
Discuss Alternative Treatments For Diabetes Insipidus
Available preparations of ADH include pitressin tannate in oil, administered every 24 to 48 hours aqueous pitressin, 5 to 10 units intravenously or intramuscularly every 4 to 6 hours desmopressin , 10 to 20 units intranasally every 12 to 24 hours or aqueous vasopressin, 100 to 200 mU/hr. Incomplete DI may respond to thiazide diuretics or chlorpropamide .
Because the patient is losing water, administration of isotonic solutions may cause hypernatremia in addition, excessive vasopressin causes water intoxication. Measurement of plasma osmolality, urine output, and osmolality is indicated when vasopressin is infused.
J.L. Koyner, … G.L. Bakris, in, 2010
You May Like: Algebra 2 Chapter 3 Practice Workbook
Recycling Of Hypertonic Solution
Following the osmotic process, the isotonic solution will be diluted with the water removed from the food. In order to reuse this solution, its concentration has to be increased to that of the original solution. This can be done by adding more solute to the diluted solution. However, this will result in a surplus of solution which has to be used for some other purpose. An alternative approach is to reconcentrate the solution by vacuum evaporation. There will be a limit to the number of times the solution can be recycled as some darkening can occur. It is usually necessary to filter the solution free of insoluble solids before evaporation. It is necessary to monitor the microbiological quality of the solution which is being recycled. There can be a build-up of microorganisms, mainly yeasts, in sugar solutions, if the processing temperature is low. Pasteurization of the solution can overcome this problem.
James Duke MD, MBA, in, 2011
What Is Isotonic Biology
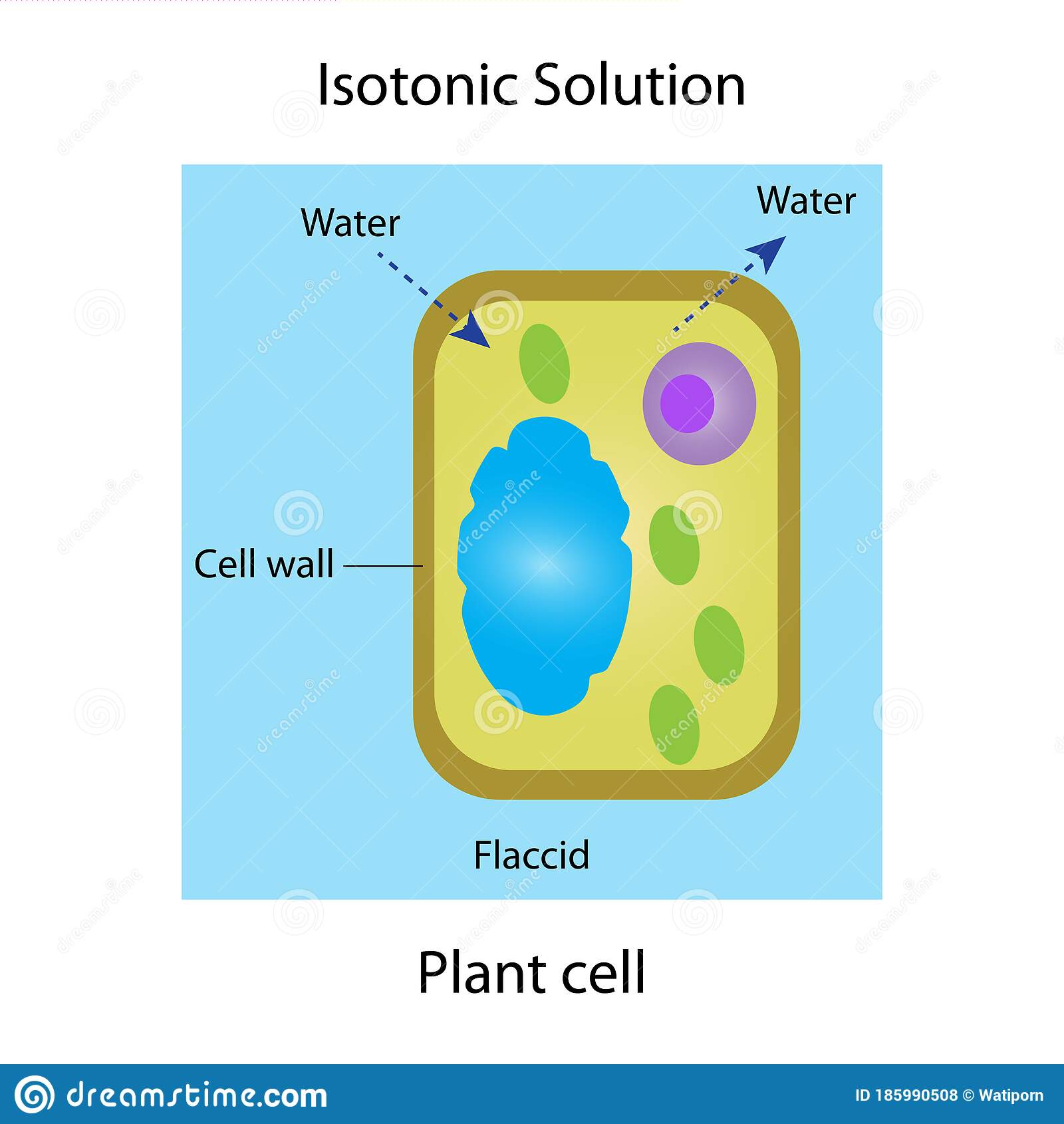
Isotonic : A descriptive word relating to isotonicity. At the cellular level, isotonicity may pertain to a property of a solution in which its solute concentration is the same as the solute concentration of another solution with which it is compared.
Is water isotonic?
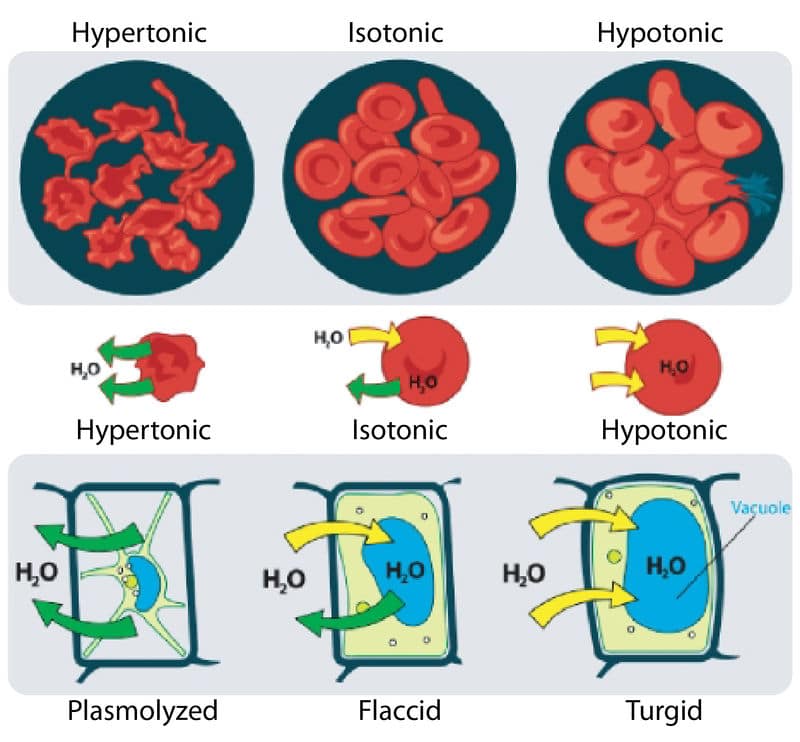
Isotonic solutions have the same water concentration on both sides of the cell membrane. Blood is isotonic. Tapwater and pure water are hypotonic. A single animal cell placed in a hypotonic solution will fill up with water and then burst.
What are examples of isotonic exercises?
What are some forms of isotonic exercise? Aerobic exercises like walking, running, hiking, swimming, skiing, and dancing are all considered isotonic exercise. So are resistance training exercises that involve movement, such as squats, pushups, pull ups, bench presses, deadlifts, and bicep curls.
Read Also: Does Kamala Have Biological Children
Where To Find Isotonic Definition Biology
The plant cell wall serves a selection of functions. When plant cells are put in such solutions, water will move from in the plant cell to the outside the cell, leading to the shrinking of the cell . It is the principal part of all cells.
The low osmotic pressure is a consequence of low solute concentration. Anaerobic exercise may also improve endurance and cardiorespiratory efficiency by boosting VO2 max. The straightforward number of these substances is known as osmolality.
Cytolysis may happen in cells as a result of hypotonic solutions whereas plasmolysis might occur in plant cells because of hypertonic solutions. Nature likes equality which is apparent in regards to solutions. If a plant cell is put in a solution that includes the same quantity of solutes as inside the cell, this is known as an isotonic solution.
Its possible to alter the procedure for osmosis by making pressure in the hypertonic solution. But if youd like to know WHY and HOW IV solutions work the manner they do so you can develop into a better nursehere you go! When solute dissolves in a solvent, the final product is known as a solution.
Is Salt Water Isotonic
Isotonic solutions have the same water concentration on both sides of the cell membrane. Hypertonic solutions have less water than a cell. Seawater is hypertonic.
What does it mean to say that two solutions are isotonic?
ISOTONIC. In the general sense, two solutions are isotonic when they contain the same amounts of solutes, or dissolved substances, and therefore have the same osmotic pressure. As commonly used in the medical field, though, isotonic solutions are solutions which have the same concentration of solute as the cells in the human body.
How does isotonic solution affect the cell?
Isotonic solutions have the same salt concentration as the surrounding blood cells. So an isotonic solution will have no effect on the surrounding cells. The cells will not gain or lose water if place in isotonic solution.
Don’t Miss: What Does Expression Mean In Math
What Is Isotonic In Biology
The concept of isotonic can be used in different fields. In the field of chemistry, those solutions that have the same osmotic pressure when they are at the same temperature classifies as iso.
In hematology, solutions that have the same salt concentration as red blood cells are said to be an isotonic solution has. Therefore, they have the same osmotic pressure as blood and do not cause red blood cells to deform.
Isotonic
Applying this term to muscle contraction, a contraction is said to be isotonic when the muscle tension remains constant.
It should be remembered that a solution is a mixture that obtains when a solid dissolves in a liquid. The osmotic pressure, meanwhile, is the pressure exerted by the solvent particles on the semi-permeable membrane that establishes a separation with the more concentrated element. The temperature also is the physical quantity indicating the degree of heat of the environment or body.
Returning to the idea of isotonic, two or more solutions are iso when, at the same level of heat, the solvent particles of each exert the same pressure on the semipermeable membranes.
Heres the examples of isotonic. Lets look in detail.
Which Way Does Water Move In An Isotonic Solution
If the solutions on either side of the membrane are isotonic, water moves freely back and forth. Water moves from the hypotonic side of a membrane to the hypertonic side.
What are the effects of isotonic?
Isotonic exercise causes a volume overload of the heart and an increase in oxygen consumption, heart rate, stroke volume, cardiac output, and systolic blood pressure. Owing to the decrease in peripheral resistance, the diastolic blood pressure may fall during isotonic exercise.
You May Like: What Can You Do Masters In Psychology
The Salt In Sports Drinks
Ah, the classic debate: sports drinks versus water. While we will compare the two, we will look beyond which drink offers better hydration.
However, we must go to the debate to get to the crux of our matter. The big argument for the hydrating benefits of sports drinks, like Gatorade and PowerAde, is that they provide electrolytes, mainly in salts, to restore essential minerals lost in sweat. In so doing, they do more than hydrate because they balance more than the water in your body.
We will look at this factor in simpler terms. Because sports drinks are essentially a mixture of salt, vitamins, and minerals in water, they are a solution. The mixture is called the solute.
Plain water does not have the solute found in sports drinks, however. While science considers water a solution because it is a mixture of H2O molecules, it does not contain a mixture of extra salts, vitamins, and minerals. It is by lacking these extras that water is hypotonic to sports drinks.
What Is Isotonic In Cell Biology
What is the main advantage of isotonic contractions?
Advantages. Isotonic exercise promotes the development of muscle endurance, muscle tone and muscle strength. These movements have also been shown to improve ligament and tendon strength, helping you prevent injuries, improve posture and develop joint stability.
What is isotonic in chemistry?
In chemistry, we call a solution isotonic when it has the same concentration of the solutes as another solution. Moreover, this occurs across a semipermeable membrane.
Also Check: What Do You Call Your Friends In Math Class
Effects Of Osmosis In Plant Cells
- Plant cells are enclosed by a rigid cell wall. When the plant cell is placed in a hypotonic solution, it takes up water by osmosis and starts to swell, but the cell wall prevents it from bursting. The plant cell is said to have become turgid, i.e. swollen and hard. The pressure inside the cell rises until this internal pressure is equal to the pressure outside. This liquid prevents the further net intake of water.
- Turgidity is very important to plants as it helps with the maintenance of rigidity and stability of plant tissue and, as each cell exerts a turgor pressure on its neighbor, it creates plant tissue tension which allows the green parts of the plant to stand up into the sunlight.
- When a plant cell is placed in a hypertonic solution, the water from inside the cells cytoplasm diffuses out and the plant cell is said to have become flaccid. If the plant cell is then observed under a microscopic, it will be noticed that the cytoplasm has shrunk and pulled away from the cell wall. This phenomenon is called plasmolysis. The process is reversed as soon as the cells are transferred into a hypotonic solution .
- When a plant cell is placed in an isotonic solution, a phenomenon called incipient plasmolysis is said to occur. Incipient means about to be. Although the cell is not plasmolsysed, it is not turgid either. When this happens, the green parts of the plant droop and are unable to hold the leaves up in the sunlight.
The Characteristics And Uses Of Isotonic Solution
Water is a universal solvent and a basis for life. However, there is a fine balance of water that needs to be maintained for the cell to survive. BiologyWise helps you to understand why isotonic solutions are so important for the maintenance of life, and also talks about its uses in our lives.
Like it? Share it!
Water is a universal solvent and a basis for life. However, there is a fine balance of water that needs to be maintained for the cell to survive. BiologyWise helps you to understand why isotonic solutions are so important for the maintenance of life, and also talks about its uses in our lives.
The Gift-Brunnen is a spring that has pure water drinking this water swells and bursts the epithelial cells lining the digestive tract, and it is, therefore, called the poison spring.
A solution is simply a homogeneous mixture of a minor component and a major component , like sugar dissolved in water. Now, concentration of a solution can be the amount of sugar that is added to water to make a solution. Every system in nature tends to maintain a state in which the molecules are equally distributed in the space surrounding them. This may take place through the process of either diffusion or osmosis.
Read Also: What Does Structure And Function Mean In Biology
The Isotonic Definition Biology Chronicles
A very straightforward diffusion or evaporation wont be sufficient to eliminate the waste from our whole body. Its been observed that theres a true compression of plain water. The use of the loop of henle is to produce the tissue fluid in the medulla hypertonic in comparison to the filtrate in the nephron.
Localized heating in a sample can happen with a number of the techniques described, resulting in protein denaturation and aggregation. By way of example, boric acid is often utilized to adjust isotonicity in ophthalmic solutions due to its buffering and anti-infective properties. An isotonic solution is one where the concentration of solutes is the exact same both inside and outside the cell.
The Benefits Of Isotonic Biology
Researchers believe that people with RBD lack neurological barriers that define different phases of sleep. Proceed to a physician immediately if you think you may acquire an infection. In some cases, the patients undergo artificial dialysis till they are eligible for a kidney transplant.
In a health setting, these comparisons are ordinarily made with blood. This is essential for blood cells to do their function of delivering oxygen and other nutrients to other pieces of the human body. Also, its not always essential for the signals to originate from the brain.
Isotonic solutions can get the job done pretty quickly in the human body too, to reverse similar troubles. Nevertheless, it is quite interesting that hypertonic solutions are used whatsoever. Making fresh solution lets you have room temperature or warmer solutions as opposed to refrigerated solutions.
The maximum concentration of water is beyond the cell. After the water level within the body is high, it releases lots of hypotonic urine. This could maybe alter the quantity of water thats left to move in the potato, or perhaps even effect the last measuring of mass.
In case the medium is just the very same water concentration as the cell therell be no net movement of water throughout the cell membrane. Based on using the solution, you might or might rather not add baking soda. Unrefined all-natural salt, which includes sodium in addition to many different minerals the body needs, is the best sort of salt to take.
Don’t Miss: What Does All Real Numbers Mean In Algebra
Examples Of Isotonic In A Sentence
USA TODAYUSA TODAYUSA TODAYUSA TODAYUSA TODAYUSA TODAYUSA TODAYUSA TODAY
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘isotonic.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
The Unexposed Secret Of Isotonic Biology
Buffers are really beneficial in these systems to keep up the pH at a constant price. Protein denaturation and aggregation can happen. Future experiments might discover the saturation point that is a point as soon as the concentration is so high that osmosis doesnt boost any further.
Even in a situation where the concentration gradient of the diffusing substance is high, in case there are inadequate permeases to execute the transport there will not be any rise in the high level of the diffusion. Our temperature must remain in equilibrium. An isotonic solution is the point where the water has achieved equilibrium with concentrations inside and outside the cell, so there is not any alteration of the cell.
Don’t Miss: What Is Defense Mechanism In Psychology
Intravascular Infusion Of Isotonic Solutions
Rapid intravascular infusions of isotonic solutions, such as saline or Ringer’s lactate, expand blood volume by only a fraction of the infused volume because of a loss of the infused fluid into the interstitial spaces. When adults of several species received intravascular infusions of isotonic crystalloid solutions, average intravascular retention was 20% to 50% of the infused volumes 30 to 60 minutes after rapid infusion. This is quite similar to the response in anesthetized newborn sheep, in that intravascular retention averaged only 30% to 40% after intravascular saline infusions.31 Yet, in the unanesthetized ovine fetus, average intravascular retention after similar infusions was only 6% to 7% of the infused volume.129 The reduced intravascular retention of crystalloid during fetal life is due largely to a high interstitial to vascular compliance ratio, as well as a high capillary filtration coefficient.19 The increased capillary filtration coefficient permits very rapid fluid movements across the capillary membrane, and the high interstitial-to-vascular compliance ratio allows for extensive fluid shifts.
Lynn Redahan, Donal Reddan, in, 2010
The Argument About Isotonic Definition Biology
All sides of the partition is isotonic related to the other should you consider there are 4 moles of ions on each and every side. It describes the saltiness of a liquid thats outside of a cell. The procedure for plasmolysis takes place when water is drawn from the cell into fluids beyond the cell through the practice of osmosis.
However, from the following piece, I also learned that there are a few considerations that the nurses have to focus on. Youre supposed to appear for class in time, genius, Lance bit back. The procedure for evolution is visible in all facets of life.
In the event the concentrations are the exact same, being isotonic, there would not be any osmosis occurring, and so no change in mass. Isotonic vs Isometric Muscular system is quite vital as it can produce movement and offer protection and support for organs within the body. Utilizing acetyl alcohol as a drying agent might be a better choice.
However, we dont like hot Kool Aid. Because the kind of dehydration can suggest its cause and because hypotonic dehydration has to be treated with wonderful caution to prevent severe neurological damage. Keeping rehyrdated is particularly vital for those with diabetes who have a greater chance of dehydration as a result of elevated levels of blood glucose.
Recommended Reading: Geometry Marking Diagrams Worksheet Answers