What Happens If You Touch Calcium Metal
Calcium will react with water or moisture causing heat. If calcium comes in touch with any parts of body and eyes, it causes irritation and corrosion
For any queries contact our BYJUS mentors.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
What Does Metallurgy Mean In Chemistry
Metallurgy definition can be given as, the branch of chemistry that deals with the extraction of metal in their pure form from their ore. Metallurgy process involves the refining of metals and the production of alloys of metals.
What is the definition of metallurgist?
Definitions of metallurgist. an engineer trained in the extraction and refining and alloying and fabrication of metals. synonyms: metallurgical engineer. examples: Sir Henry Bessemer.
Metallurgy: Definition Principle Process And Solved Questions
Content Curator| Updated On -Oct 10, 2022
Metallurgy is the process of extracting pure metal from its ore. Naturally, most of the metals are found in their combined state. So it becomes essential to extract the pure form of metal for various other domestic and commercial purposes.
|
Table of Content |
Read Also: Material Properties and Concepts of metals and non metals
Minerals from which metals can be easily extracted are called Ores. The ore that is mined from the earth usually contains earthy impurities such as dirt, sand and rocks. These impurities are called Gangue. Once the gangue is removed, the ore is subjected to a further purification process to remove the impurities.
You May Like: What Does Aq Mean In Chemistry
What Is Metal Or Non
A metal is a component, material, or alloy that is usually solid, transparent, shiny, and has good conductivity in terms of electrical and thermal. Metals are usually malleable, which ensures that they can be indefinitely hammered or pressed without fracturing or cracking, as well as fusible or ductile
Is Metallurgical Engineering Related To Chemistry
Thus metallurgy is a full -fledged branch of Engineering and is not a branch of Chemistry.
What is metallurgy definition and process?
Metallurgy Definition & Processes. What is Metallurgy? Metallurgy is defined as a process that is used for the extraction of metals in their pure form. The compounds of metals mixed with soil, limestone, sand, and rocks are known as minerals.
What is the importance of Chemical Metallurgy?
Chemical metallurgy is chiefly concerned with the reduction and oxidation of metals, and the chemical performance of metals. Subjects of study in chemical metallurgy include mineral processing, the extraction of metals, thermodynamics, electrochemistry, and chemical degradation .
What are the key steps involved in the metallurgy of metals?
The key steps involved in the metallurgy of metals are: Crushing and grinding of the ore Concentration of the ore Extraction of the crude metal
What is the difference between mechanical and physical metallurgy?
In contrast, physical metallurgy focuses on the mechanical properties of metals, the physical properties of metals, and the physical performance of metals. Topics studied in physical metallurgy include crystallography, material characterization, mechanical metallurgy, phase transformations, and failure mechanisms.
Recommended Reading: What Does Anticyclone Mean In Geography
What Is Metallurgy Furnace
A metallurgical furnace, more commonly referred to as a furnace, is a device used to heat and melt metal ore to remove gangue, primarily in iron and steel production. There are several different types of furnaces used in metallurgy to work with specific metal and ores.
What is the meaning of metallurgical Industry?
Definition. Industry concerned with the extraction, refining, alloying and fabrication of metals.
Frequently Asked Questions Faq
Q 1. What is the heat treatment of a metal?Answer: Heat treatment is a technique used to modify the strength, ductility, toughness, hardness, and corrosion resistance of metals. Annealing, precipitation strengthening, quenching, and tempering are typical heat treatment procedures. For superior characteristics and more effective material processing, mechanical and thermal treatments are frequently combined to create what is known as thermo-mechanical treatments. High-alloy special steels, superalloys, and titanium alloys all go through these procedures.
Q 2. What is the difference between roasting and calcination?Answer: Calcination is the method of heating the ore below its melting point in a limited supply of air. This method is used for oxide and carbonate ores. For example, when the concentrated ore of zinc carbonate is heated, it produces zinc oxide and carbon dioxide is released.
ZnCO3ZnO+CO2
Roasting, on the other hand, is the process of heating the ore in an excess supply of air below its melting point. This method is applied to sulphide ores. For example, when the zinc sulphide ore is heated in the presence of oxygen, it is converted into zinc oxide and sulphur dioxide gas is produced.
ZnS+O2ZnO+SO2
There are occasions when hydrometallurgical procedures can be performed directly on the ore without any prior preparation. The ore must frequently undergo multiple stages of mineral processing as a pretreatment.
Related Topics
Also Check: What The Bleep Do We Know Quantum Physics
Refining And Purification Of Obtained Metal
This method is used for ores refining and purifications.
Liquation:- Liquation processes are used when metal and impurity have a different boiling point.
Cupellation:- Cupel is made up of bone ash and cement mixture. Cupel is so strong and even can tolerate 4000 °C temperature. This is a method used to remove the impurities Pb from precious metals Au and Ag.
Distillation:- Distillation is used for the metal having a low boiling point and melting point. eg. Zn, Hg.
Polling:- This method is used when the main impurities with metal are its own oxide. Metal separated from metal oxide by a reduction for reduction we will use a reducing agent.
CH4 + MO CO/CO2 + H2O + M
Electro Refining :- This method is used for ultra purification of metals, Anode MUD generally contains less basic metals like silver and gold which can be recovered from the meet of cost of refining.
Zone Refining:- This method is used for semiconductor Si, Ge. This method also gives ultra-pure metals. Impurities are more soluble in a zone of higher temperatures.
Vapour phase refining:- In this method the is converted into its volatile compounds and collected elsewhere, it is then decomposed to give pure metal. Two requirements are Metal Should form a voltaic compound. voltaic compounds should be easily decomposed.
What Is Another Term For Chemical Metallurgy
The science and technology of extracting metals from ores and refining them. Also known as process metallurgy.
Is metallurgy a chemical or physics?
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are called alloys.
Also Check: What Does Region Mean In Geography
Physical Properties Of Metals
- All the metals are good conductors of heat and electricity. Cooking utensils and irons are made up of metals as they are good conductors of heat.
- Ductility is the ability of the material to be stretched into a wire. This ability allows metals to be drawn into wires and coupled with their durability, find applications as cable wires and for soldering purposes. Because Metal can be drawn into wires we can say that metals are ductile.
- Malleability is the property of substances which allows them to be beaten into flat sheets. Aluminium sheets are used in the manufacturing of Aircrafts because of their lightweight and strength. Other metal sheets are used in automobile industries, for making utensils, etc. Therefore, metals are malleable.
- Metals are sonorous because they produces a deep or ringing sound when struck with another hard object.
- Usually, all the metals have a shiny appearance but these metals can also be polished to have a shiny appearance.
Why Is Purification Of Extracted Metals Needed
The metal obtained from the extraction of metal from ore is frequently impure. It frequently contains impurities such as carbon, silicon, phosphorus, and so on.
The method of refining any metal is determined by its nature and the nature of its impurities. As a result, various methods are employed in this regard. The electrolytic method is the most important method and yields very pure metal.
Also Check: Geometry Dash The Secret Box
Is Metallurgy Part Of Chemistry
The entire scientific and technological process used for isolation of the metal from its ore is known as metallurgy. The extraction and isolation of an element from its combined form involves various principles of chemistry. Still, some general principles are common to all the extraction processes of metals.
Is metallurgy a branch of physical chemistry?
Metallurgical and material engineering is a sub branch of chemical engineering.
What are physical chemistry chapters for NEET?
- Physical Chemistry Class 11 Chapter 1 Some Basic Concepts of Chemistry.
- Physical Chemistry Class 11 Chapter 2 Structure of an atom.
- Physical Chemistry Class 11 Chapter 3 States of matter: Gases and liquids.
- Physical Chemistry Class 11 Chapter 4 Thermodynamics.
Is metallurgy important for JEE mains?
Yes, why not, every year 2- 3 questions are asked in JEE. Metallurgy is a kind of chapter which can give you complete comprehensions .. And also one of the most logical chapters of IC So you may expect a more than one choice .
What is meant by metallurgical industry?
The metallurgical industry can be broadly divided into primary and secondary metal production operations. Primary refers to the production of metal from ore.Secondary refers to production of alloys from ingots and to recovery of metal from scrap and salvage.
Who invented metal material?
S In Metallurgical Process
The various processes involved in extracting metals from their ores and refining them for use are referred to as metallurgy.
The following are the various steps in the metal extraction or metallurgical process:
- Crushing and grinding the ore.
- The concentration of ore, is also known as ore enrichment.
- Metal extraction from concentrated ore.
- Impure metals are refined or purified.
Copper Flash Smelting Process
Recommended Reading: What Is Human Geography The Study Of
What Kind Of Societies And Professional Organizations Do Metallurgists Have
- ASM International, founded as the American Society for Metals in 1913, is the largest association for metals-oriented materials scientists. ASM offers professional development courses and certification in metallography. It issues well-known materials standards, organizes databases and publishes journals, and hosts online communities for colleges and universities and emerging professionals.
- The Society for Mining, Metallurgy, & Exploration organizes an Environmental Division and a Mineral & Metallurgical Processing Division. It provides networking opportunities through its divisions and an annual conference. It also organizes a mentor program and student chapters. It shares technical information by publishing books, magazines, and journals and managing a database of articles. It offers e-learning opportunities, funds awards, and hosts an online Student Center.
*2020 US Bureau of Labor Statistics salary figures and job growth projections for mining and geological engineersreflect national data not school-specific information. Conditions in your area may vary. Data accessed September 2021.
Conversion Of Ores Into Metal Oxide
All concentrated ores are not easily reduced so concentrated ores must be converted into a form that is suitable for a reduction. Usually, sulfides and other ores are converted into oxides by following two processes. Calcination Roasting
Calcination:- Calcination is the heating of the ores in absence of . It is commonly done for oxy-ores like carbonate and sulfate.
MCo2 heat Mo + Co2
Calamine heat ZnO + Co2
Anglesite heat PbO + So3
Roasting:- Roasting is heating ores in presence of air. It is commonly done for non-oxy ores like sulfate ore. By roasting, sulfate ores can be converted to more reactive oxide for some ores partial roasting is done which is followed by self-reduction of ores.
Read Also: What Does V Mean In Math
Uses Of Metals And Alloys
- Engineering metals typically include zinc, titanium, nickel, magnesium, iron, copper, chromium, aluminium and silicon. These metals are typically utilised in alloys, with the notable exception being silicon. There has been a lot of focus on studying the iron-carbon alloys, which comprises cast irons and steels.
- Plain carbon steels are used in applications that call for high strength and low cost since they are weight and corrosion resistant. Cast iron is a part of the iron-carbon system that includes ductile iron. Iron-manganese-chromium alloys are used in directional drilling, a non-magnetic application .
- Materials like copper alloys, titanium alloys, nickel alloys, galvanised and austenitic stainless steel and stainless steel are used to resist corrosion.
- Aluminum and magnesium alloys are often utilised when a strong, lightweight component is required, like in aerospace and automotive applications. Monel and other copper-nickel alloys are used in non-magnetic applications and very corrosive environments. Nickel-based superalloys like Inconel are used in high-temperature applications such as heat exchangers, pressure vessels, turbocharges and gas turbines.
- To lessen creep, single crystal alloys are employed at very high temperatures. Integrated circuits and Metal-oxide-silicon transistors in modern electronics require high purity single crystal silicon.
Concentration Of The Ore
The ores are extracted from the mines and there are pieces of stone, pebbles, pebbles, sand, and stones of pollutants. These residues are called gangueor matrix. The process of separating gangues present in ores obtained from mines is called concentrating of ore.
To concentrate the ore, firstly by separating large pieces of ore by hand. After this, they are broken into small pieces by handcuffs. These small pieces are put in the crushers. The crushers are like a millstone. Which consists of two plates.
One plate runs and the other plate stays level. The crushers crumble small pieces. The small pieces obtained in this way puts pieces in stamp mill. Where there are frequent hammer injuries on them. This process grinds ore into a very fine form.
Several methods can be used after grinding large pieces of ore to concentrate the ore. Depending on the nature of the ore and the gangue, one or more of these methods is sometimes used. Some of the following are the major methods.
Recommended Reading: How To Solve Geometry Problems Step By Step
Which Is The Best Definition Of Metallurgy
Definition of metallurgy : the science and technology of metals : a science that deals with the nature and uses of metal : the science of obtaining metals from their ores and preparing them for use
Which is the best definition of Metals Science?
n. 1. The science that deals with procedures used in extracting metals from their ores, purifying and alloying metals, and creating useful objects from metals. 2. The study of metals and their properties in bulk and at the atomic level.
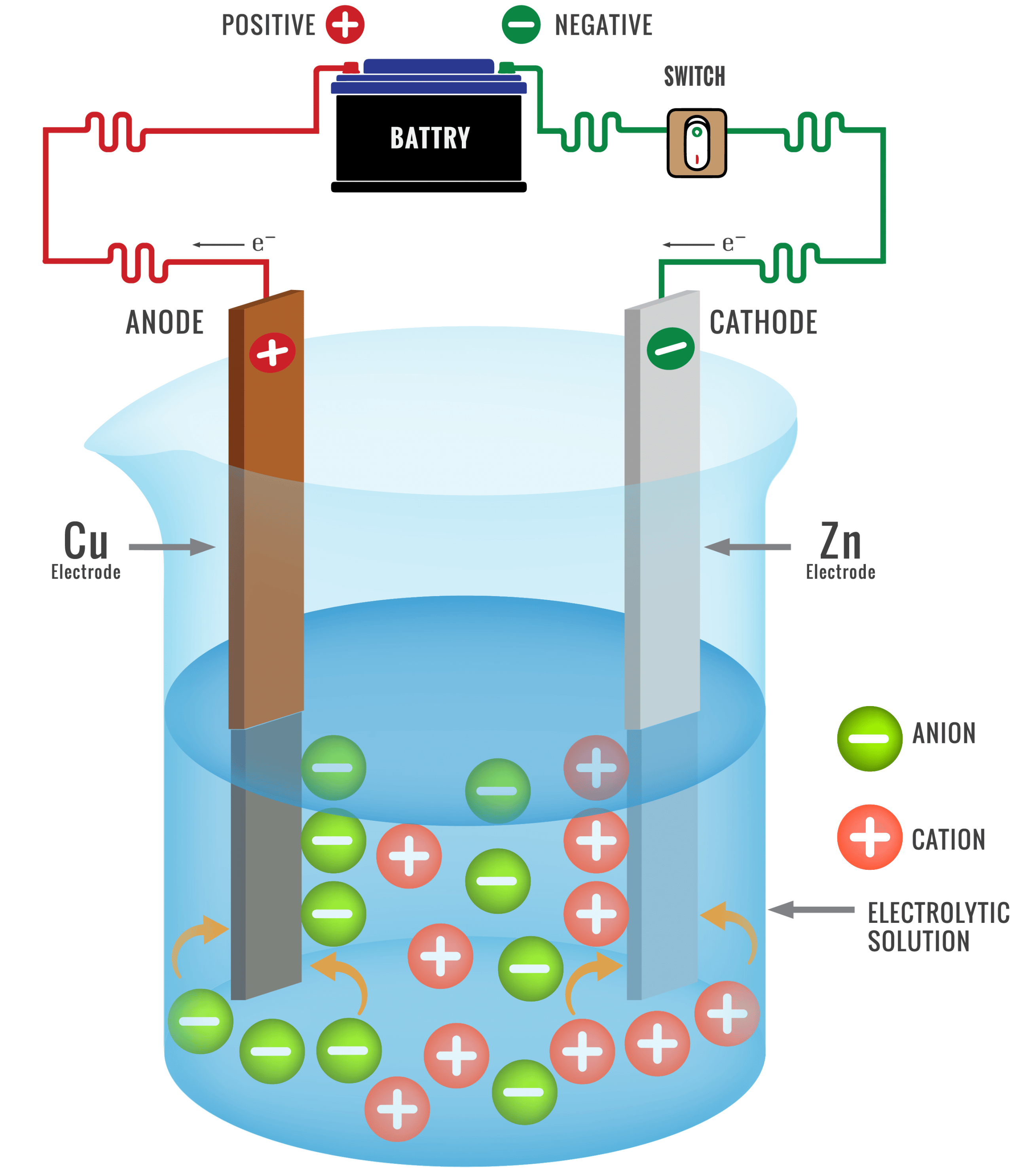
Purification Of The Metal
The metal obtained from the extraction of the metal from the ore is often impure. It often contains impurities of carbon silicon phosphorus etc.
The method of refining of any metal depends on its nature and the nature of impurities present in it. Therefore, different types of methods are used for this. Electrolytic method is main in this. Very pure metal is obtained in this method.
The electrolytic method of refining metals can be explained by the example of refining of copper metal by this method. For the refining of copper metal, electrolyte solution of copper metal salts in electrolytic cell acts as an electrolyte, thick plates of impure metal act as anode and thin plates of pure metal act as cathode.
When electrolysed in an acidic solution of copper sulphate, thick plates of impure copper metal at the anode begin to dissolve and impurities accumulate down the solution. These impurities are called anode mud. Cu forms its cations that move into the solution.
Also Check: Fundamental Theorem Of Affine Geometry
What Are The Types Of Metallurgical Process
Roasting, smelting and converting are the most common pyrometallurgical processes. Pyrometallurgical processes of course require significant energy input to reach the desired high temperatures during the process. This energy is usually provided by combustion, exothermic reaction of the material, or electrical heat.
Is Metallurgy A Physics

While chemical metallurgy involves the domain of reduction/oxidation of metals, physical metallurgy deals mainly with mechanical and magnetic/electric/thermal properties of metals treated by the discipline of solid state physics.
What is ore in chemistry?
Ores are naturally occurring rocks that contain metals or metal compounds in sufficient amounts to make it worthwhile extracting them. The method used to extract a given metal from its ore depends upon the reactivity of the metal and so how stable the ore is.
Why do we study physical metallurgy?
Physical metallurgy: Links the structure of materials with their properties. Concepts such as alloy design and microstructural engineering help link processing and thermodynamics to the structure and properties of metals. Through these efforts, goods and services are produced.
Who is a metallurgist?
Metallurgists study the properties of metals and then apply their findings to practical applications, such as metal production. They work with a range of metals including copper, precious metals, iron, steel, zinc and aluminium alloys.
How do you say metallurgist?
What is the difference between physical metallurgy and metallurgy?
Chemical or extractive metallurgy is concerned with the extraction of metals from ores and with the refining of metals. Physical metallurgy is concerned with the physical and mechanical properties of metals as affected by composition, mechanical working, and heat treatment.
What is a synonym for metallurgy?
Recommended Reading: Gina Wilson All Things Algebra Geometry Answer Key
What Are The Steps In The Metallurgy Process
The key steps involved in the metallurgy of metals are: Crushing and grinding of the ore Concentration of the ore Extraction of the crude metal
How are metals extracted from minerals in metallurgy?
Metals are commercially extracted from minerals at low cost and minimum effort. These minerals are known as ores. A substance which is added to the charge in the furnace to remove the gangue is known as flux. Metallurgy deals with the process of purification of metals and the formation of alloys.