Covalent Bonds Form By The Sharing Of Electrons
All the characteristics of a cell depend on the molecules it contains. A is defined as a cluster of atoms held together by here electrons are shared between atoms to complete the outer shells, rather than being transferred between them. In the simplest possible a molecule of hydrogen two H atoms, each with a single , share two electrons, which is the number required to fill the first shell. These shared electrons form a cloud of negative charge that is densest between the two positively charged nuclei and helps to hold them together, in opposition to the mutual repulsion between like charges that would otherwise force them apart. The attractive and repulsive forces are in balance when the nuclei are separated by a characteristic distance, called the bond length.
A further crucial property of any bondcovalent or noncovalentis its strength. Bond strength is measured by the amount of energy that must be supplied to break that bond. This is often expressed in units of kilocalories per , where a kilocalorie is the amount of energy needed to raise the temperature of one liter of water by one degree centigrade. Thus if 1 kilocalorie must be supplied to break 6 × 1023 bonds of a specific type , then the strength of that bond is 1 kcal/mole. An equivalent, widely used measure of energy is the , which is equal to 0.239 kilocalories.
Some energies important for cells. Note that these energies are compared on a logarithmic scale.
What Are The Six Main Elements In Living Organisms
Six elements on the periodic table account for 97 percent of your body’s mass: carbon, hydrogen, nitrogen, oxygen, sulfur and phosphorus. Not coincidentally, these elements exist in great abundance in the Milky Way galaxy and beyond. Human beings are, as a popular saying suggests, stardust.
The names of these six elements can be remembered using the acronym CHNOPS. They are not distributed uniformly throughout the body, but some of them concentrate preferentially in some tissues.
The Significance Of Carbon
A compound found mainly in living things is known as an organic compound. Organic compounds make up the cells and other structures of organisms and carry out life processes. Carbon is the main element in organic compounds, so carbon is essential to life on Earth. Without carbon, life as we know it could not exist.
Recommended Reading: Define Abiotic Biology
What Are The Major Chemical Elements Found In Cells In Biology
The cells of living things are made mainly of four elements: carbon, hydrogen, oxygen and nitrogen. They make up 96% of the atoms that are in living things, so they would be considered major chemicals. However, depending on how you define major, other elements that only make up a few percent of cells can top the list. If major also means essential for life, then trace elements are very major though they make up just 0.5% of the atoms in an organism.
TL DR
The four most important elements in cells are carbon, hydrogen, oxygen and nitrogen. However, other elements — like sodium, potassium, calcium and phosphorus — are also important.
Sugars Provide An Energy Source For Cells And Are The Subunits Of Polysaccharides
The simplest the monosaccharidesare compounds with the general formula n, where n is usually 3, 4, 5, 6, 7, or 8. Sugars, and the molecules made from them, are also called carbohydrates because of this simple formula. Glucose, for example, has the formula C6H12O6 . The formula, however, does not fully define the : the same set of carbons, hydrogens, and oxygens can be joined together by covalent bonds in a variety of ways, creating structures with different shapes. As shown in , for example, can be converted into a different mannose or galactosesimply by switching the orientations of specific groups relative to the rest of the molecule. Each of these sugars, moreover, can exist in either of two forms, called the d-form and the l-form, which are mirror images of each other. Sets of molecules with the same chemical formula but different structures are called , and the subset of such molecules that are mirror-image pairs are called optical isomers. Isomers are widespread among organic molecules in general, and they play a major part in generating the enormous variety of sugars.
An Outline of Some of the Types of Sugars Commonly Found in Cells.
The reaction of two monosaccharides to form a disaccharide. This reaction belongs to a general category of reactions termed condensation reactions, in which two molecules join together as a result of the loss of a water molecule. The reverse reaction
Recommended Reading: Is Paris Jackson Michael Real Daughter
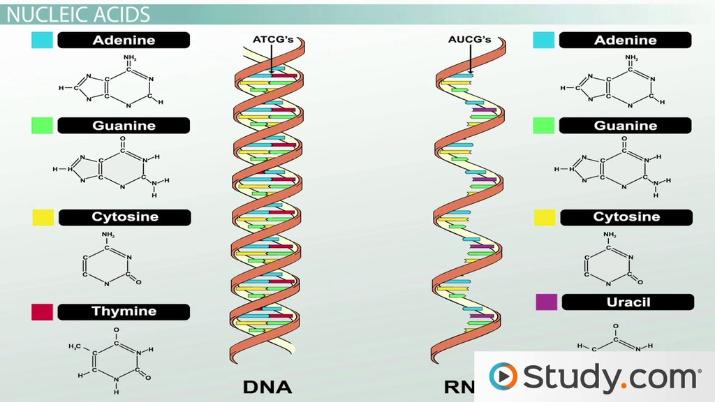
Function Of Nucleic Acids In Cells
The main function of nucleic acids is to store and carry the hereditary information for the functioning of the cell.
The nucleic acids include two major classes of biological molecules, deoxyribonucleic acid and ribonucleic acid , and consist of nucleotides.
Protein and nucleic acid enzymes catalyze biochemical reactions in both catabolism and anabolism of macromolecules.
Catabolism – the breakdown of biomolecules in living organisms.
Anabolism – the synthesis of complex biological macromolecules.
One of the basic qualities of organic compounds – to possess a variety of properties, depends, in particular, on their ability to form different structures or isomers.
Isomers are macromolecules with the same molecular formula but different chemical structures.
There are two main types of structures of organic compounds:
Structural isomers of macromolecules differ in the placement of their covalent bonds.
Examples of structural isomers is biological molecules of carbohydrates – glucose and fructose. Because of their different structures, they have different properties and are metabolized differently.
Stereoisomers have similar placements of their covalent bonds but differ in how these bonds are made to the surrounding atoms. Stereoisomers can be geometrical or optical.
Geometrical isomers can have different physical, but similar chemical properties.
Examples of geometrical isomers are glucose and galactose.
What Does Chnops Stand For In Biology
The term CHNOPS is a mnemonic acronym for the six main chemical elements that make up living things. They are carbon , hydrogen , nitrogen , oxygen , phosphorus and sulphur .The term CHNOPS is a mnemonic acronym for the six main chemical elements that make up living things. They are carbon , hydrogen , nitrogen , oxygen , phosphorus , not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.
Don’t Miss: What Does Abiotic Mean
Van Der Waals Interactions
Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. They occur between polar, covalently bound, atoms in different molecules. Some of these weak attractions are caused by temporary partial charges formed when electrons move around a nucleus. These weak interactions between molecules are important in biological systems.
The Chemical Components Of A Cell
Matter is made of combinations of elementssubstances such as hydrogen or carbon that cannot be broken down or converted into other substances by chemical means. The smallest particle of an element that still retains its distinctive chemical properties is an atom. However, the characteristics of substances other than pure elementsincluding the materials from which living cells are madedepend on the way their atoms are linked together in groups to form molecules. In order to understand how living organisms are built from inanimate matter, therefore, it is crucial to know how all of the chemical bonds that hold atoms together in molecules are formed.
You May Like: Unit 1 Test Study Guide Geometry Basics
How Did Rna Evolve Into Dna
In modern metabolism, protein-based enzymes called reverse transcriptases can copy RNA to produce molecules of complementary DNA. … In the second, the RNA world contained RNA polymerase ribozymes that were able to produce single-stranded complementary DNA and then convert it into stable double-stranded DNA genomes.
Amino Acids Polymerize By Eliminating The Elements Of Water
The product has ends with different properties.
- An end with a free amino group this is called the amino terminal or N-terminal.
- An end with a free carboxyl group this is called the carboxyl terminal or C-terminal.
Conventions for writing sequences of amino acids.
Abbreviations for the amino acids are usually used most of the three letter abbreviations are self-evident, such as gly for glycine, asp for aspartate, etc.
There is also a one-letter abbreviation system it is becoming more common. Many of the one-letter abbreviations are straightforward, for example:
- G = glycine
Others require a little imagination to justify:
- F = phenylalanine .
- Y = tyrosine .
- D = aspartate .
Still others are rather difficult to justify:
- W = tryptophan .
- K = lysine
Question: What do you suppose “Q” represents?
You should be aware this is becoming more and more commonly used, and you should have the mindset of picking it up as you are exposed to it, rather than resisting.
Sequences are written with the N-terminal to the left and the C-terminal to the right.
Although R-groups of some amino acids contain amino and carboxyl groups, branched polypeptides or proteins do not occur.
The sequence of monomer units in a macromolecule is called the PRIMARY STRUCTURE of that macromolecule. Each specific macromolecule has a unique primary structure.
THE REGULAR REPEAT OF MONOMER UNITS HAVING THE SAME SIZE AND THE SAME BOND ANGLES LEADS TO HELICAL POLYMERS.
Read Also: Unit 1 Homework 4 Angle Addition Postulate
What Elements Can You Find At Home
What are 5 pure elements that can be found in your home?
- Argon and tungsten are in incandescent light bulbs.
- Mercury is in some thermostats and in switches in space heaters that turn off when tipped over.
- Copper is used in electrical wiring and in some water pipes.
- Carbon is in pencils.
- Phosphorous is on the tips of matches and ignites from the friction of striking them.
Some Polar Molecules Form Acids And Bases In Water
One of the simplest kinds of chemical , and one that has profound significance in cells, takes place when a possessing a highly between a hydrogen and a second atom dissolves in water. The hydrogen atom in such a molecule has largely given up its to the companion atom and so exists as an almost naked positively charged hydrogen in other words, a . When the polar molecule becomes surrounded by water molecules, the proton is attracted to the partial negative charge on the O atom of an adjacent water molecule and can dissociate from its original partner to associate instead with the oxygen atoms of the water molecule to generate a hydronium . The reverse reaction also takes place very readily, so one has to imagine an state in which billions of protons are constantly flitting to and fro from one molecule in the solution to another.
Acids in water. The reaction that takes place when a molecule of acetic acid dissolves in water. Water molecules are continuously exchanging protons with each other to form hydronium and hydroxyl ions. These ions in turn rapidly recombine to form
Because the of a hydronium can be passed readily to many types of molecules in cells, altering their character, the concentration of H3O+ inside a cell must be closely regulated. Molecules that can give up protons will do so more readily if the concentration of H3O+ in solution is low and will tend to receive them back if the concentration in solution is high.
You May Like: Subfields In Psychology Worksheet Answers
Macromolecular Interactions Involving Proteins
If covalent links exist then the structure is not considered quaternary. In proteins with quaternary structure the deaggregated subunits alone are generally biologically inactive.
Here are some examples of quaternary structure.
Quaternary structure in proteins is the most intricate degree of organization considered to be a single molecule. Higher levels of organization are multimolecular complexes.
Biological Function Of Carbohydrates
Plants and algae produce millions of tons of carbohydrates each year through photosynthesis.
The main function of carbohydrates is to provide energy, particularly through glucose.
During cellular respiration, glucose is broken down and oxidized within cells. This process is used to synthesize adenosine triphosphate the source of energy for cellular reactions.
When the quantity of adenosine triphosphate are sufficient, simple carbohydrates are converted to carbohydrate polymers or fat and stored.
Carbohydrates also have other important functions in all living organisms.
For example, they serve as building materials within the plant cells and perform cell-to-cell identification when attached to the external surfaces of the cytoplasmic membrane.
Read Also: Michael Jackson Children Biological Father
Water Is The Most Abundant Substance In Cells
Water accounts for about 70% of a cell’s weight, and most intracellular reactions occur in an environment. Life on Earth began in the ocean, and the conditions in that primeval environment put a permanent stamp on the chemistry of living things. Life therefore hinges on the properties of water.
In each water the two H atoms are linked to the O atom by covalent bonds . The two bonds are highly because the O is strongly attractive for electrons, whereas the H is only weakly attractive. Consequently, there is an unequal distribution of electrons in a water molecule, with a preponderance of positive charge on the two H atoms and of negative charge on the O . When a positively charged region of one water molecule comes close to a negatively charged region of a second water molecule, the electrical attraction between them can result in a weak bond called a . These bonds are much weaker than covalent bonds and are easily broken by the random thermal motions due to the heat energy of the molecules, so each bond lasts only an exceedingly short time. But the combined effect of many weak bonds is far from trivial. Each water molecule can form hydrogen bonds through its two H atoms to two other water molecules, producing a network in which hydrogen bonds are being continually broken and formed . It is only because of the hydrogen bonds that link water molecules together that water is a liquid at room temperature, with a high boiling point and high surface tensionrather than a gas.
How Is Chnops Useful In Our Study Of The Chemistry Of Living Things
The CHNOPS elements come together to form biomolecules, the molecules found in all of the living organisms on earth. Carbon provides the structural framework for biomolecules. Hydrogen, nitrogen, and oxygen are common in living organisms because they bond easily with the carbon and are abundant in nature.
Read Also: Algebra 2 Chapter 4 Test Form A
The Importance Of Water
Water is made of two hydrogen atoms bond to an oxygen atom. Though water exists as separate molecules and does not form physical connections with proteins, lipids, carbohydrates and nucleic acids, it is essential for life. The molecules that make life possible only work if they are dissolved in water. Enzymes speed up chemical reactions, lipids serve as energy stores and sugars are easily broken down to make energy, but all of this is possible because these molecules are floating in a watery environment. The hydrogen and oxygen in water are two of the big four elements of life, but these two serve a distinct purpose as water, compared with the purposes they serve when they are part of the carbon-containing organic molecules.
Related Articles
Monosaccharides Can Polymerize By Elimination Of The Elements Of Water
If two anomeric hydroxyl groups react the product has no reducing end . This is the case with sucrose
If the anomeric hydroxyl reacts with a non-anomeric hydroxyl of another sugar, the product has ends with different properties.
- A reducing end .
- A nonreducing end.
Since most monosaccharides have more than one hydroxyl, branches are possible, and are common.Branches result in a more compact molecule.If the branch ends are the reactive sites, more branches provide more reactive sites per molecule.
Let’s now turn to nucleotides and nucleic acids.
Read Also: What Is Michael Jackson’s Daughter’s Name
Amino Acids Are The Subunits Of Proteins
Amino acids are a varied class of molecules with one defining property: they all possess a carboxylic group and an , both linked to a single carbon atom called the -carbon . Their chemical variety comes from the that is also attached to the -carbon. The importance of amino acids to the cell comes from their role in making , which are polymers of amino acids joined head-to-tail in a long chain that is then folded into a three-dimensional structure unique to each type of . The covalent between two adjacent amino acids in a protein chain is called a the chain of amino acids is also known as a . Regardless of the specific amino acids from which it is made, the polypeptide has an amino group at one end and a carboxyl group at its other end . This gives it a definite directionalitya structural polarity.
A small part of a protein molecule. The four amino acids shown are linked together by three peptide bonds, one of which is highlighted in yellow. One of the amino acids is shaded in gray. The amino acid side chains are shown in red. The two ends of a
Like sugars, all amino acids, except glycine, exist as optical in d- and l-forms . But only l-forms are ever found in proteins . The origin of this exclusive use of l-amino acids to make proteins is another evolutionary mystery.