Drawing The Lewis Structure For Xef2
Video: Drawing the Lewis Structure for XeF2
For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table. Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet . We also need to check to make sure we only used the number of available valence electrons we calculated earlier.
There are a total of 22 valence electrons in the Lewis structure for XeF2.
The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons.
It is helpful if you:
- Try to draw the XeF2 Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to XeF2 for more practice.
How Can Noble Gases Combine To Form Compounds Such As Xef2 Krf2 Xeof2 Since They Have Their Octets Full They Should Not Combine At All Please Explain Their Formation And Their Lewis Structure
Priya G N answered this
The electromagnetic attractive force from its nucleus is weaker for the electrons in Xenon’s outer shell than for the equivalent electrons in the smaller-sized noble gases. Plus, it has more electrons flying around than those other smaller nobles, and they are all putting repulsive forces on each other, helping to weaken the strength of the bond. Therefore, the really really powerful oxidizers like Fluorine and Chlorine can actually steal one of the Xenon’s electrons out from under it and arrange itself to be in a more stable state than the Xe was before.
The electrons in the valence shell of Xe and Kr become unpaired and these are promoted to vacant 5d orbitals. Then on the basis of bond pairs and lone pairs the molecule gets particular geometry.
The structures of oxy fluorides of xenon are explained by hybridization.
Let us write the Lewis structure of above compounds.
- 0
Sf4 Bond Angles And Shape
The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. Fluorine atoms on the equatorial positions have the bond angles of 102 degrees, and the axial ones have 173 degrees, which are a little different than the trigonal bipyramidal molecular geometry leading to a see-saw shape.
The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms.
Concluding Remarks
To conclude all the properties we can say that,
- Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure.
- There are three lone pairs on each fluorine atom.
- It has a molecular geometry of the formula AX4E it forms a see-saw shape and has a trigonal bipyramidal molecular geometry.
- SF4 has sp3d hybridization and is polar in nature.
About Priyanka
To read, write and know something new every day is the only way I see my day! Well, that rhymed. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. Having an MSc degree helps me explain these concepts better. I write all the blogs after thorough research, analysis and review of the topics. And if not writing you will find me reading a book in some cosy cafe!View all posts by Priyanka
You May Like: Glencoe Geometry Answers Chapter 7
Lewis Structure For Xef2
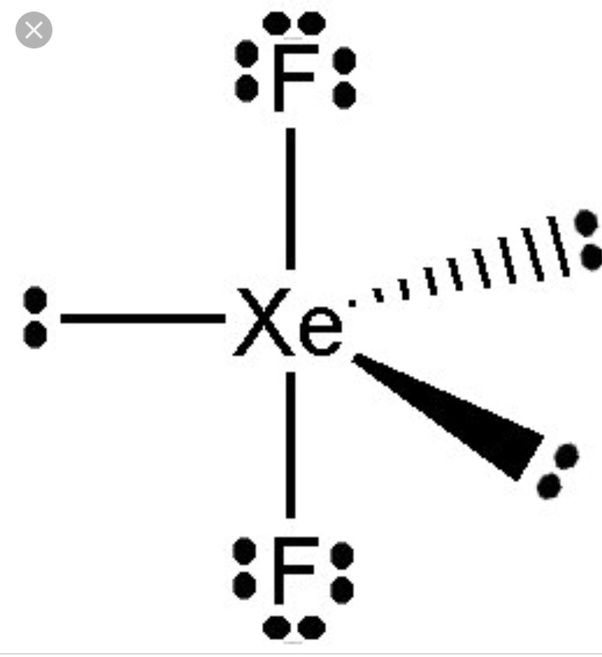
Atomization energies at 0 K and heats of formation at 0 and 298 K are predicted for XeF3+, XeF3â, XeF5+, XeF7+, XeF7â, and XeF8 from coupled cluster theory ) calculations with effective core potential correlation-consistent basis sets for Xe and including correlation of the nearest core electrons. Additional corrections are included to achieve near chemical accuracy of ±1 kcal/mol. XeF2 structure features two covalent bonds between one xenon atom and two fluorine atoms. The xenon atom also holds 3 lone pairs of electrons. Physical Properties of Xenon Difluoride – XeF 2. Odour: Nauseating odour: Appearance: White solid: Covalently-Bonded Unit: 1: Hydrogen Bond Acceptor: 2: Complexity : 2.8: Solubility: Insoluble in water: Uses of Xenon Difluoride – XeF 2. Used to.
Is Xef2 Polar Or Nonpolar
Xenon difluoride is a chemical compound with chemical formula as XeF2. It is considered as a strong fluorinating agent. It is also one of the most stable compounds of Xenon. It is also a moisture-sensitive substance like other inorganic covalent compounds. Many students may have doubts regarding whether XeF2 is polar or not. In this article, we will discuss this in detail and will also cover its properties and applications.
So, Is XeF2 Polar or Nonpolar? XeF2 is nonpolar in nature because of its linear-shaped geometry having fluorine atoms symmetrically on both sides of the xenon atom. However Xe-F bond is polar because the electronegativity of Xe and F is different but the polarity of both Xe-F bonds gets canceled by each other resulting in a nonpolar XeF2 molecule.
Xenon difluoride exists as a dense white solid at room temperature. It is one of the most stable compounds of the Xenon family.
It decomposes on contact with vapors and light, but if stored properly, it is a stable substance.
It was discovered in the year around 1962.
The crystal structure of the XeF2 substance contains parallel units of XeF2 connected with each other.
The molecular mass of Xenon difluoride is calculated as below:
Mol mass of XeF2 = 1* 131 + 2 * 55.8 = 169.29 g·mol1.
If we talk about the chemical composition of XeF2, the molecule contains 2 atoms of Fluorine and 1 atom of Xenon.
The Xenon is a central atom surrounded by Fluorine atoms on its both side connected to Xenon through covalent bonds.
Also Check: Introduction To Psychology Hawkes Learning
F What Is The Molecular Shape Of The Molecule
Don’t forget about the age old question of How does polarization affect water’s chemical interactions?
Don’t forget about the age old question of What is a systemic level of analysis?
If you want to learn more check out What are the five primary pruposes of racist violence?
If you want to learn more check out Cite examples of Hominoids.
Question : What Is The Total Number Of Valence Electrons In The Lewis Structure Of Xef2
Atomic number of Xe is 54 and its Electronic configuration is-
5s24d105p6
There are 8 valence electrons in Xe.
Atomic number of F is 9 and its Electronic configuration is-
2s22p5
There are 7 valence electrons in one F atom.
As there is one Xe atom and two F atoms in XeF2 the total number of valence electrons is = 8+ = 22.
Don’t Miss: Unit 1 Test Study Guide Geometry Basics Answer Key
Why Is Xef2 A Nonpolar Molecule
Xenon difluoride consists of two fluorine atoms and one xenon atom. Xe and F atoms form covalent bonds with each other.
Being more electronegative, the fluorine atom attracts the bonded electron pair slightly more towards it and gains a partial negative charge.
On the other hand, the xenon atom gains a partial positive charge.
Due to the difference between the electronegativity of fluorine and xenon atom, the molecule of XeF2 ensures non zero dipole moment originating in the direction of fluorine.
The dipole moment of a molecule is the measure of its polarity.
Both Xe-F atoms have equal dipole moment but in the opposite direction of each other due to which the net dipole moment of the entire molecule turns out to be zero.
Therefore, the Dipole moment of XeF2 is 0 D.
Have a look at the below image of the geometrical structure of the XeF2 molecule.
Factors Affecting Polarity Of A Molecule
Electronegativity: It is termed as the strength of an atom to attract the bonded pair electrons towards its side. The higher electronegative atom attracts the bonded electrons slightly more towards its side.
If there is a difference between the electronegativity of two atoms forming a covalent bond, the polarity rises in such bonds.
The polarity of a molecule is directly proportional to the difference between the electronegativity of two atoms forming that molecule.
Geometrical shape: The shape of a molecule is an important parameter to check whether a molecule is polar or not.
The symmetrically shaped molecules are nonpolar in nature whereas the molecule that is asymmetric is polar in nature.
Dipole Moment: The molecules polarity is measured by its dipole moment. It is denoted by D. It SI unit is Debye.
The molecules that are nonpolar have 0 dipole moment value.
It is a vector quantity ie have magnitude as well as direction.
D = Q * R
You May Like: What Does Span Mean In Linear Algebra
Use Lewis Structure Guidelines To Draw The Lewis Structure Of Phosphate Ion
Lewis structure and shape of xef2. Iii State the molecular shape 1 mark iv What is the hybridization or give the hybrid orbital type 2 mark. Whats the molecular geometry of. SCN- is an anion having a chemical name Thiocyanate.
How to find the shape of XeF 2 using VSEPR theory. Draw lewis structure of BrF5 and XeF2 using VSPER theory. The shape of the molecule refers only to the arrangement of the bonds.
What is the meaning of Lewis structure. Lewis Dot Structure of XeF2. Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry for xenon difluoride XeF2.
Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom. Considering Xenon is a Nobel Gas XeF2 is a stable structure. Lewis structures also called electron-dot structures or electron-dot diagrams are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
The structure is square planar and consists of the non-bonding electrons forming an octahedral shape. Xef2 Vsepr Gallery southeasternedu. Crystalline solid used for fluorinating purposes in electrochemical procedures and The Lewis structure of a given chemical compound is crucial for knowing all the.
Predic molecular shape and hybridization on central atom. Ii Identify the number of lone pairs 1 mark. I also go over hybridization shape and bond angle.
Xef2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride.
If we look at the process of synthesis of xenon difluoride, heres the equation:
Xe + F2 Heat> XeF2
XeF2 acts as an oxidizing and fluorinating agent and is used to oxidize different hydrocarbons including both aromatic and acyclic compounds.
Not only this, but this fluoride compound can also be used to etch silicon to form silicon tetrafluoride without any external energy application.
If you are thinking about what XeF2 looks like, it appears as a colorless-to-white crystalline solid with a density of around 4.32 g/cc.
This halide can cause some serious hazards like skin burns and major eye damage. Not only this, if inhaled or swallowed, it turns out to be fatal.
Recommended Reading: Algebra 1 Fsa Review
Xef4 Polarity Is Xef4 Polar Or Nonpolar
Although the bonds between Xenon and Fluorine atoms are polar, XeF4 is a nonpolar molecule. Wondering how? All the Xe-F bonds are in opposition with each other mutually, making the sum of dipole moment zero. As there are four electrons on the Xenon atom, which are localized as nonbonding pairs of electrons. As the overall arrangement of the atoms and electrons in the molecule is such that the vector sum of the dipoles is zero, XeF4 is a nonpolar molecule.
Concluding Remarks
Xenon Tetrafluoride is one of those molecules that is relatively easy to understand. Its Lewis structure is one of the least complicated structures, as all the Fluorine atoms are arranged in the symmetric pattern. The lone pairs in the molecule are located in a perpendicular plane in an octahedral shape to keep their repulsive forces at a minimum.
To summarize this blog post, we can say that XeF4 has 36 valence electrons. It has two lone pairs of nonbonding electrons on the central atom of Xenon. The molecule has octahedral electron geometry and square planar molecular geometry. XeF4 is a nonpolar molecule and has sp3d2 hybridization.
At the Geometry of Molecules, we like knowing what you think. So let us know your thoughts on this molecule in the comments below.
About Priyanka
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. It has a chemical formula of SF2 and can be generated by the reaction of Sulphur Dioxide and Potassium Fluoride or Mercury Fluoride. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape.
| Name of molecule | |
| No of Valence Electrons in the molecule | 20 |
Don’t Miss: Geometry Dash Practice Music
What Is The Hybridization Of Xenon Difluoride
In the hybridization of xenon difluoride, Xenon is the central atom. Now if we count the number of valence shell in Xe we will find two electrons in the 5s orbital and six electrons in the 5p orbital. Its ground state electronic configuration will be 5s2 5p6. However, in the excited state, its configuration will change to 5s2 5p5 5d1. All in all, in the excited state configuration 5s,5py, 5px, 5pz,5dzx atomic orbitals of the xenon atom will be hybridized to forms 5 sp³d hybrid orbitals.
Two-hybrid orbitals are used in the formation of F-Xe-F sigma bonds by overlapping the two half-filled 2pz atomic orbitals of fluorine. The other remaining three hybrid orbitals contain the lone pairs which do not participate in bonding.
What Is The Molecular Geometry Of Xef3
4.5/5geometrygeometry
Furthermore, does XeF3 exist?
Xe has a complete filled 5p configuration. As a result when it undergoes bonding with an odd number of F atoms it leaves behind one unpaired electron. This causes the molecule to become unstable. As a result XeF3 and XeF5 do not exist.
Likewise, what is the structure of XeF2? XeF2 structure features two covalent bonds between one xenon atom and two fluorine atoms. The xenon atom also holds 3 lone pairs of electrons.
Also to know, what is the molecular geometry of XeF4?
XeF4 Molecular Geometry And Bond AnglesThe lone pairs of Xenon lie in the perpendicular plane in an octahedral arrangement. Therefore, XeF4 molecular geometry is square planar. The bond angles are 90 or 180°. The lone pairs lie on the opposite sides of the molecule basically at 180° from each other.
What is the Lewis structure for ICl4?
Drawing the Lewis Structure for IClIn the Lewis structure of ICl4– there are total of 36 valence electrons. Since Iodine is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4– the Iodine atom has 12 valence electrons.
Don’t Miss: Beth Thomas Married
Question : What Is The Xef2 Lewis Structure
XeF2 has a total of 22 valence electrons, 8 from Xe and 7 from each F. Xe being a less electronegative atom is the central atom and it forms a single bond with each F atom. Therefore, Xe doesnt follow the octet rule. Each fluorine atom with 7 valence electrons forms a single bond with Xe completing their octet. Each dot represents an electron. A pair of dots between two atomic symbols represents a bond pair. Other pairs of dots on atomic symbols represent lone pairs.
Question 2: What is the chemical name for XeF2 ?
XeF2 has xenon as a central atom singly bonded to two fluorine atoms. The name of the compound is Xenon difluoride.
Is Sf2 Polar Or Nonpolar
To determine the polarity of any molecule, we check for the following factors:
- Presence of lone pairs
- The shape of the molecule
- The difference in electronegativities of atoms
- Net Dipole moment in the molecule
Sulfur Difluoride has a bent molecule geometry having two single bonds and two lone pairs of electrons. These lone pairs of electrons distort the shape of the molecule, and hence it is non-linear. As these lone pairs try to keep their repulsive forces minimal, they push down the Fluorine atoms.
Due to the presence of the lone pairs, there is symmetry in the molecule. And as a result, the charges will not be evenly distributed, increasing the chances of the polarity in the molecule.
When we compare Sulphur and Fluorine atoms electronegativities, the value of electronegativity of Sulphur is 2.58 and for Fluorine is 3.98. So here the difference of the electronegativities of both these atoms is much higher than 0.5, which makes the S-F bonds polar. And due to this vast difference in electronegativity, there will be a dipole moment between Sulphur and Fluorine atoms. The direction of the dipole moment will be from the Sulphur atom towards the Fluorine atom, as here Fluorine will try to pull the shared electrons to itself.
Concluding Remarks
To summarise this blog, we can say that,
About Priyanka
Recommended Reading: Accuracy And Precision Worksheet Answer Key
Lewis Dot Structure Of Xef2
XeF4 Lewis Structure. Now that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to draw its Lewis structure. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms. The bonds in the structure are shown using lines, whereas the electrons not participating in the bond formation.