What Is The Heat Of Vaporization
As we provide heat to a liquid, its energy increases, which results in an increase in the overall temperature. At the boiling point, the additional heat is used up by the molecules to overcome the intermolecular force of attraction in the liquid and change to the gaseous state.
When 1 mole of liquid is transformed into a gaseous state, the amount of heat provided by this process is known as the Heat of Vaporization.
Elevation Of The Boiling Point Of A Solvent
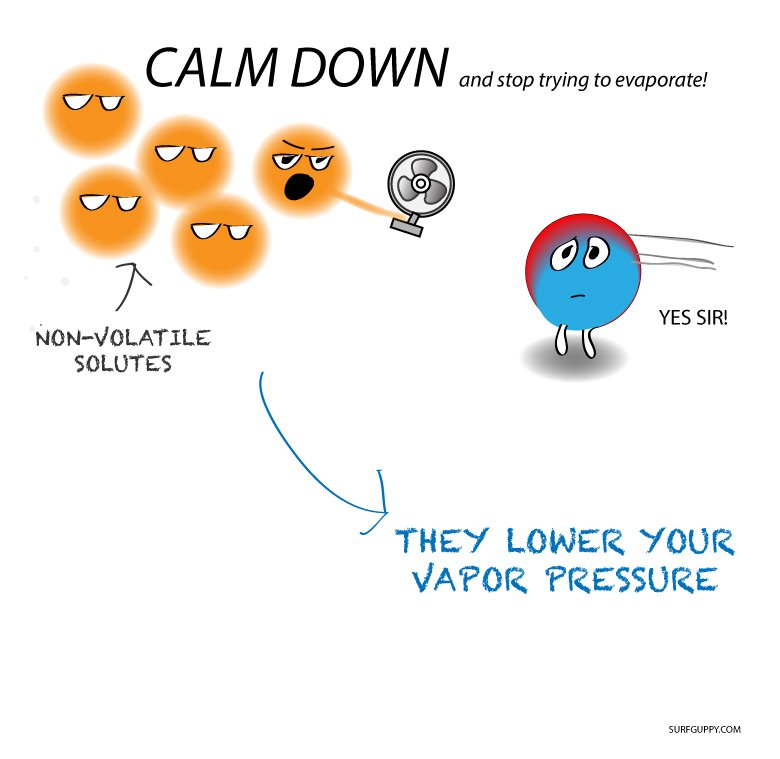
As described in the chapter on liquids and solids, the boiling point of a liquid is the temperature at which its vapor pressure is equal to ambient atmospheric pressure. Since the vapor pressure of a solution is lowered due to the presence of nonvolatile solutes, it stands to reason that the solutions boiling point will subsequently be increased. Compared to pure solvent, a solution, therefore, will require a higher temperature to achieve any given vapor pressure, including one equivalent to that of the surrounding atmosphere. The increase in boiling point observed when nonvolatile solute is dissolved in a solvent, Tb, is called boiling point elevation and is directly proportional to the molal concentration of solute species:
where Kb is the boiling point elevation constant, or the ebullioscopic constant and m is the molal concentration of all solute species.
Boiling point elevation constants are characteristic properties that depend on the identity of the solvent. Values of Kb for several solvents are listed in Table 2.
| Solvent |
|---|
| 8.1 |
| Table 2. Boiling Point Elevation and Freezing Point Depression Constants for Several Solvents |
Reverse Osmosis Water Purification
In the process of osmosis, diffusion serves to move water through a semipermeable membrane from a less concentrated solution to a more concentrated solution. Osmotic pressure is the amount of pressure that must be applied to the more concentrated solution to cause osmosis to stop. If greater pressure is applied, the water will go from the more concentrated solution to a less concentrated solution. This is called reverse osmosis. Reverse osmosis is used to purify water in many applications, from desalination plants in coastal cities, to water-purifying machines in grocery stores , and smaller reverse-osmosis household units. With a hand-operated pump, small RO units can be used in third-world countries, disaster areas, and in lifeboats. Our military forces have a variety of generator-operated RO units that can be transported in vehicles to remote locations.
Figure 9.Figure 10.
Don’t Miss: Theory Of Everything 2 Music
Liquid Mixtures: Raoult’s Law
Raoult’s law gives an approximation to the vapor pressure of mixtures of liquids. It states that the activity of a single-phase mixture is equal to the mole-fraction-weighted sum of the components’ vapor pressures:
- P
^}} is the vapor pressure of component i . Raoult’s law is applicable only to non-electrolytes it is most appropriate for non-polar molecules with only weak intermolecular attractions .
Systems that have vapor pressures higher than indicated by the above formula are said to have positive deviations. Such a deviation suggests weaker intermolecular attraction than in the pure components, so that the molecules can be thought of as being “held in” the liquid phase less strongly than in the pure liquid. An example is the azeotrope of approximately 95% ethanol and water. Because the azeotrope’s vapor pressure is higher than predicted by Raoult’s law, it boils at a temperature below that of either pure component.
There are also systems with negative deviations that have vapor pressures that are lower than expected. Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components. Thus, the molecules are “held in” the liquid more strongly when a second molecule is present. An example is a mixture of trichloromethane and 2-propanone , which boils above the boiling point of either pure component.
What Did Caesar Say
De Bello Gall by Julius Caesar. Image from Wikimedia.
Latin is an interesting and useful language, even if you dont want to read Julius Caesars writings. A knowledge of Latin helps us understand our own language better. Take the colligative. Where did that come from? If you know a little Latin, you know that it comes from two Latin words meaning to tie together. This helps you better understand some of the science terminology we use every day.
Recommended Reading: Holt Geometry Chapter 7 Test Form A Answers
Boiling Point Of Water
Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by the Antoine equation:
- log
| 20 |
Relation To Boiling Point Of Liquids
As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points. This is illustrated in the vapor pressure chart that shows graphs of the vapor pressures versus temperatures for a variety of liquids. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, 760 Torr, 101.325 kPa, or 14.69595 psi.
For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point , which is where the vapor pressure curve of methyl chloride intersects the horizontal pressure line of one atmosphere ” rel=”nofollow”> atm) of absolute vapor pressure.
Although the relation between vapor pressure and temperature is non-linear, the chart uses a logarithmic vertical axis to produce slightly curved lines, so one chart can graph many liquids. A nearly straight line is obtained when the logarithm of the vapor pressure is plotted against 1/ where T is the temperature in degrees Celsius. The vapor pressure of a liquid at its boiling point equals the pressure of its surrounding environment.
Also Check: Who Is Paris Jackson’s Real Father
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Vapor Pressure Of Water
The vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. If we raise the pressure and keep the temperature, the water will condense.Have a look at this handy vapor pressure for water table to find the pressure for different temperatures quickly:
| T |
|---|
Don’t Miss: Define Movement In Geography
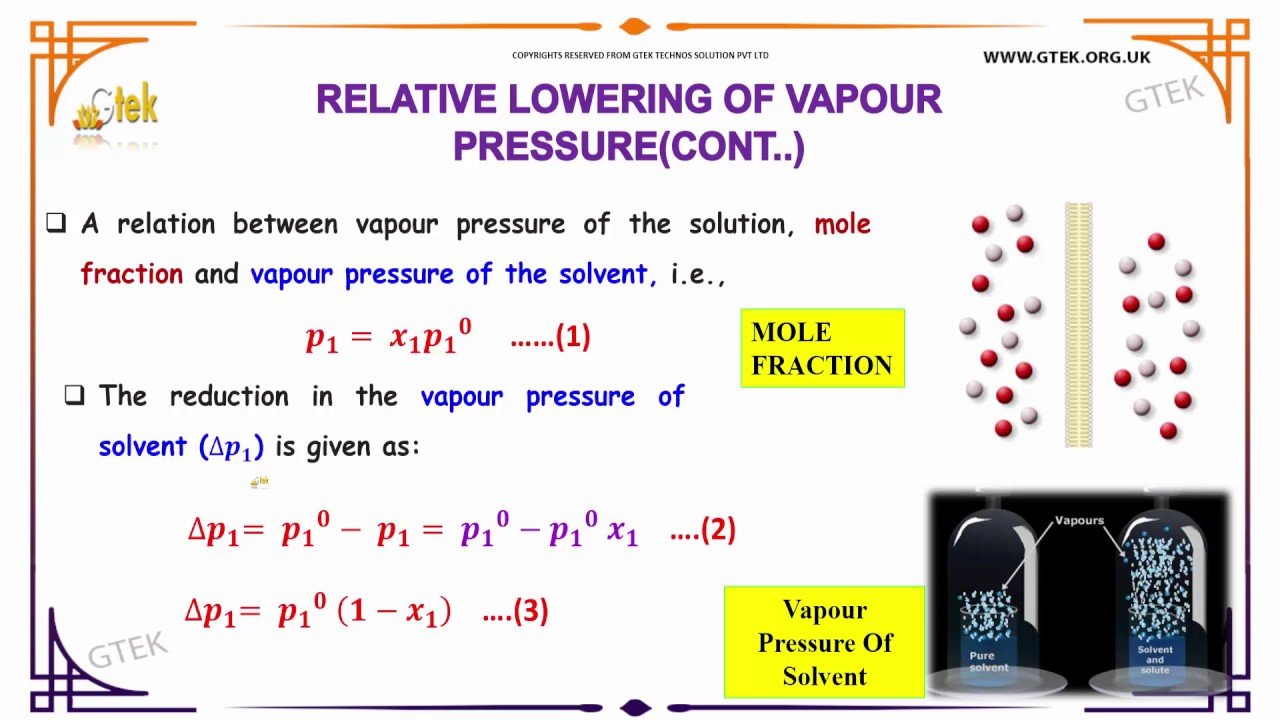
Ideal Solution And Deviations From Raoult’s Law
Ideal solutions are those which follow Raoult’s Law. Suppose the molecules of solvent and solute are represented by a and b. Now let AB represent the attractive force acting between A and B, and AA between A and A. Then, if:
ab = aa
The solution which has the same vapor pressure and is determined by Raoult’s law is determined as the ideal solution.
ab> aa
The positive deviation is defined as a molecule that escapes from the surface solution faster than the vapor pressure of the solution will become higher.
Vapor Pressure And Boiling
- What is vapor pressure?
Answer:
The equilibrium vapour pressure is typically the pressure exerted by a liquid …. it is A FUNCTION of temperature…
Explanation:
At lower temperatures, water exerts a much lower vapour pressure…but these should often be used in calculations…especially when a gas is collected by water displacement. Tables of #”saturated vapour pressure”#
Also Check: Algebra 1 Eoc 2015
Characteristics Of Vapour Pressure
However, as time passes, the number of molecules in the vapour phase increases while the rate of condensation also increases. It reaches a stage where the rate of evaporation is equal to the rate of condensation. This phase is called the stage of equilibrium.
As represented by the manometer, at this point the pressure exerted by the molecules is called the vapour pressure of the liquid. Vapour pressure is defined as the pressure exerted by the vapour present above the liquid.
Temperature is the sole factor that affects vapour pressure. The vapour pressure of a liquid is independent of the volume of liquid in the container, whether one litre or thirty litres both samples will have the same vapour pressure at the same temperature. Temperature has an exponential connection with vapour pressure, which means that as the temperature rises, the vapour pressure rises as well.
The process of evaporation depends on different factors:-
Which Has Maximum Vapour Pressure
The material with the lowest boiling point would, therefore, have the highest vapour pressure at room temperature . The highest boiling point material will have the lowest vapour pressure. Vapour pressure is an evaporation-related fluid element.
This was just a quick intro about the vapour pressure. To find out more about different states of liquid, please download BYJUS the learning app.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
You May Like: How To Calculate Half Life Of A Drug
Vapor Pressure Lowering Definition In Chemistry And Example
- Post author
Now we will discuss about the vapor pressure lowering definition chemistry. Vapor pressure lowering occurs because of a very high level of solute and evaporation didnt happen. Before we discuss vapor pressure lowering of a substance even further, lets discuss about the evaporation process itself first.
Colligative Properties Of Electrolytes
As noted previously in this module, the colligative properties of a solution depend only on the number, not on the kind, of solute species dissolved. For example, 1 mole of any nonelectrolyte dissolved in 1 kilogram of solvent produces the same lowering of the freezing point as does 1 mole of any other nonelectrolyte. However, 1 mole of sodium chloride forms 2 moles of ions when dissolved in solution. Each individual ion produces the same effect on the freezing point as a single molecule does.
Don’t Miss: What Is The Meaning Of Abiotic
Key Concepts And Summary
Properties of a solution that depend only on the concentration of solute particles are called colligative properties. They include changes in the vapor pressure, boiling point, and freezing point of the solvent in the solution. The magnitudes of these properties depend only on the total concentration of solute particles in solution, not on the type of particles. The total concentration of solute particles in a solution also determines its osmotic pressure. This is the pressure that must be applied to the solution to prevent diffusion of molecules of pure solvent through a semipermeable membrane into the solution. Ionic compounds may not completely dissociate in solution due to activity effects, in which case observed colligative effects may be less than predicted.
What Is The Vapour Pressure Of Liquid
Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with temperature. The temperature at which the vapour pressure at the surface of a liquid becomes equal to the pressure exerted by the surroundings is called the boiling point of the liquid.
Don’t Miss: Blanket Jackson Real Parents
Colligative Properties Of Relative Lowering Of Vapour Pressure
Colligative properties are defined as the properties of the solution determined by the ratio of the number of solute particles and solvent molecules in a solution. Colligative properties of a solution include relative lowering of vapor pressure, the elevation of boiling point, depression of freezing point, and osmotic pressure.
All the colligative properties are inversely proportional to the molar mass of solute for all mass ratios of solute and solvent.
Relative molar mass is determined by measuring the colligative properties of the solution of the non-ionized solute. These solutions include urea, glucose in water, etc. Osmotic pressure is also a type of colligative property that can be determined by utilizing a semi-permeable membrane.
Solution For Problem 7pe Chapter 12
Chemistry | 11th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Chemistry | 11th Edition
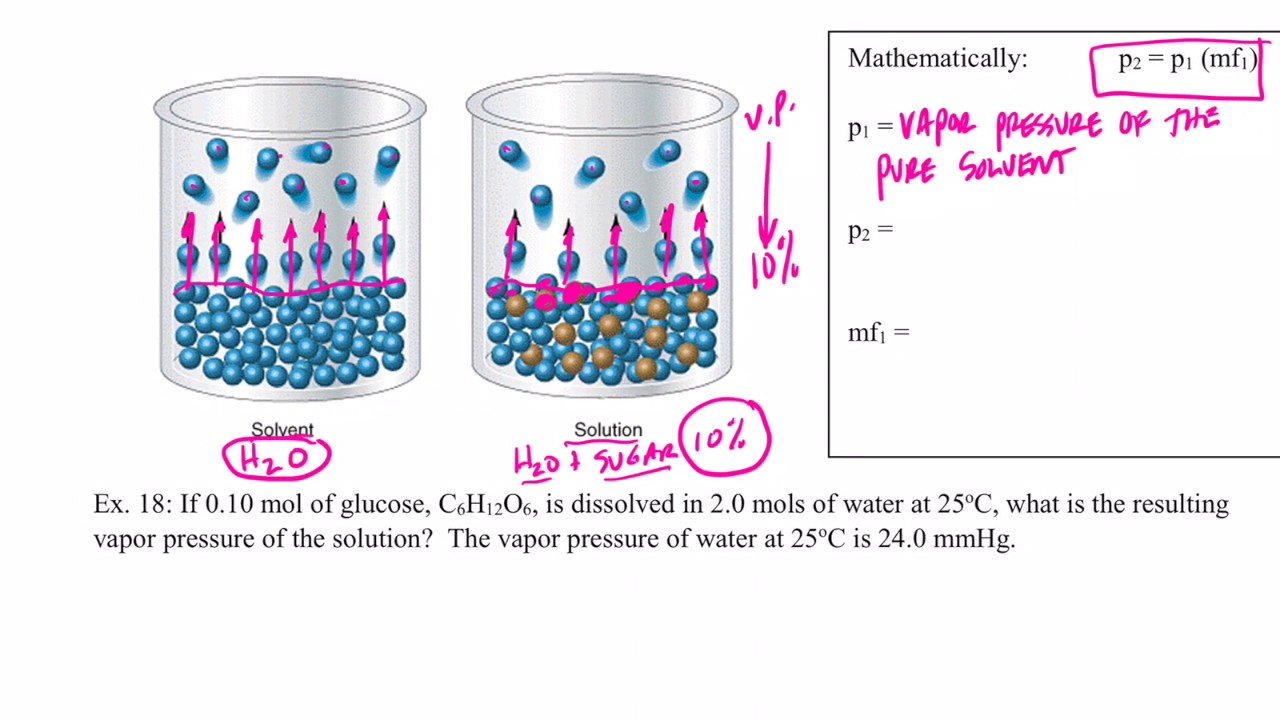
Calculate the vapor pressure of a solution made by dissolving 82.4g of urea in 212 mL of water at 35°C. What is the vapor-pressure lowering?
Step 1 of 3
According to the Raoult’s law vapour pressure of the solution can be calculated by the following formula
= Mole fraction of the solvent
= Pressure of the solution
ISBN: 9780073402680
Chemistry was written by and is associated to the ISBN: 9780073402680. This textbook survival guide was created for the textbook: Chemistry, edition: 11. Since the solution to 7PE from 12 chapter was answered, more than 1551 students have viewed the full step-by-step answer. The full step-by-step solution to problem: 7PE from chapter: 12 was answered by , our top Chemistry solution expert on 11/08/17, 03:59AM. The answer to ?Calculate the vapor pressure of a solution made by dissolving 82.4g of urea in 212 mL of water at 35°C. What is the vapor-pressure lowering? is broken down into a number of easy to follow steps, and 29 words. This full solution covers the following key subjects: pressure, vapor, Molar, made, mass. This expansive textbook survival guide covers 25 chapters, and 3257 solutions.
Other solutions
You May Like: What Does Abiotic Mean In Biology
Estimating Vapor Pressures With Antoine Equation
The Antoine equation is a pragmatic mathematical expression of the relation between the vapor pressure and the temperature of pure liquid or solid substances. It is obtained by curve-fitting and is adapted to the fact that vapor pressure is usually increasing and concave as a function of temperature. The basic form of the equation is:
Sublimations and vaporizations of the same substance have separate sets of Antoine coefficients, as do components in mixtures. Each parameter set for a specific compound is only applicable over a specified temperature range. Generally, temperature ranges are chosen to maintain the equation’s accuracy of a few up to 810 percent. For many volatile substances, several different sets of parameters are available and used for different temperature ranges. The Antoine equation has poor accuracy with any single parameter set when used from a compound’s melting point to its critical temperature. Accuracy is also usually poor when vapor pressure is under 10 Torr because of the limitations of the apparatus used to establish the Antoine parameter values.
The Wagner equation gives “one of the best” fits to experimental data but is quite complex. It expresses reduced vapor pressure as a function of reduced temperature.
Colligative Properties And De
Sodium chloride and its group 2 analogs calcium and magnesium chloride are often used to de-ice roadways and sidewalks, due to the fact that a solution of any one of these salts will have a freezing point lower than 0 °C, the freezing point of pure water. The group 2 metal salts are frequently mixed with the cheaper and more readily available sodium chloride for use on roads, since they tend to be somewhat less corrosive than the NaCl, and they provide a larger depression of the freezing point, since they dissociate to yield three particles per formula unit, rather than two particles like the sodium chloride.
Because these ionic compounds tend to hasten the corrosion of metal, they would not be a wise choice to use in antifreeze for the radiator in your car or to de-ice a plane prior to takeoff. For these applications, covalent compounds, such as ethylene or propylene glycol, are often used. The glycols used in radiator fluid not only lower the freezing point of the liquid, but they elevate the boiling point, making the fluid useful in both winter and summer. Heated glycols are often sprayed onto the surface of airplanes prior to takeoff in inclement weather in the winter to remove ice that has already formed and prevent the formation of more ice, which would be particularly dangerous if formed on the control surfaces of the aircraft .
Figure 5.
Also Check: Which Founding Contributors To Psychology Helped Develop Behaviorism
Osmosis And Osmotic Pressure Of Solutions
A number of natural and synthetic materials exhibit selective permeation, meaning that only molecules or ions of a certain size, shape, polarity, charge, and so forth, are capable of passing through the material. Biological cell membranes provide elegant examples of selective permeation in nature, while dialysis tubing used to remove metabolic wastes from blood is a more simplistic technological example. Regardless of how they may be fabricated, these materials are generally referred to as semipermeable membranes.
Consider the apparatus illustrated in Figure 7, in which samples of pure solvent and a solution are separated by a membrane that only solvent molecules may permeate. Solvent molecules will diffuse across the membrane in both directions. Since the concentration of solvent is greater in the pure solvent than the solution, these molecules will diffuse from the solvent side of the membrane to the solution side at a faster rate than they will in the reverse direction. The result is a net transfer of solvent molecules from the pure solvent to the solution. Diffusion-driven transfer of solvent molecules through a semipermeable membrane is a process known as osmosis.
Figure 7.
where R is the universal gas constant.