Corrosionpedia Explains Chemical Oxygen Demand
Chemical oxygen demand testing is typically performed using a strong oxidizing chemical. Organic matter is oxidized into carbon dioxide and water in an acidic condition. The quantity of organic matter or the demand of oxygen is calculated by determining how much oxidizing chemical was consumed during the test.
Chemical oxygen demand tests are typically performed on wastewater. The pollution level is calculated by measuring the amount of organic matter in the water. Water with too much organic material can have a negative effect on the environment in which the wastewater is discharged.
Chemical oxygen demand is similar to biochemical oxygen demand in that they are both used to calculate the oxygen demand of a water sample. The difference between the two is that chemical oxygen demand measures everything that can be oxidized, whereas biochemical oxygen demand only measures the oxygen demanded by organisms.
Key Takeaways: Hydrogen Bonds
- A hydrogen bond is an attraction between two atoms that already participate in other chemical bonds. One of the atoms is hydrogen, while the other may be any electronegative atom, such as oxygen, chlorine, or fluorine.
- Hydrogen bonds may form between atoms within a molecule or between two separate molecules.
- A hydrogen bond is weaker than an ionic bond or a covalent bond, but stronger than van der Waals forces.
- Hydrogen bonds play an important role in biochemistry and produce many of the unique properties of water.
What Happens If Water Changes Phase
|
The phase changes of water |
The changes from a liquid to a solid or to a gas are called phase changes. When a substance such as water changes phase, its physical appearance changes, but not its chemical properties. This is because the chemical structure remains the same, but the molecules of which it consists will float a little further apart. In the solid state the water molecules are fairly close together, but in the liquid state they are a bit further apart. The water becomes liquid as a result of parting molecules. When water changes from liquid to gas the molecules will part even further, that is why we cannot detect it.
You May Like: Why Am I Always Late Psychology
In What States Can Water Be Found
Water exists in three states: solid, liquid and gaseous. At a normal temperature of about 25oC it is liquid, but below 0oC it will freeze and turn to ice. Water can be found in the gaseous state above 100oC, this is called the boiling point of water, at which water starts to evaporate. The water turns to gas and is then odourless and colourless. How fast water evaporates depends on the temperature; if the temperature is high, water will evaporate sooner.
A Hydrate Quiz For Review And Fun
For each question, choose the best answer. The answer key is below.
- Aldehydes and ketones information from Michigan State University
- Information about the formation of hydrates from aldehydes and ketones from the University of Calgary
- Methane hydrate information from the U.S. Department of Energy
This content is accurate and true to the best of the authors knowledge and does not substitute for diagnosis, prognosis, treatment, prescription, and/or dietary advice from a licensed health professional. Drugs, supplements, and natural remedies may have dangerous side effects. If pregnant or nursing, consult with a qualified provider on an individual basis. Seek immediate help if you are experiencing a medical emergency.
Recommended Reading: What Does Mole Mean In Chemistry
Physical Properties Of Water
Water is a colourless and tasteless liquid. The molecules of water have extensive hydrogen bonds resulting in unusual properties in the condensed form. This also leads to high melting and boiling points. As compared to other liquids, water has a higher specific heat, thermal conductivity, surface tension, dipole moment, etc. These properties form the reason for its significance in the biosphere. Water is an excellent solvent and therefore it helps in the transportation of ions and molecules required for metabolism. It has a high latent heat of vaporization which helps in the regulation of body temperature.
What Does Chemical Oxygen Demand Mean
Chemical oxygen demand is the amount of oxygen needed to oxidize the organic matter present in water. Chemical oxygen demand testing is used to determine the amount of oxidation that will occur and the amount of organic matter in a water sample. Chemical oxygen demand testing is also used to determine the amount of inorganic chemicals in a sample.
Also Check: Geometry 11.6 Worksheet Answers
Water In Ionic Hydration Shells
Water molecules interact strongly with ions, which are electrically-charged atoms or molecules. Dissolution of ordinary salt in water yields a solution containing the ions Na+ and Cl . Owing to its high polarity, the H2O molecules closest to the dissolved ion are strongly attached to it, forming what is known as the inner or primary hydration shell. Positively-charged ions such as Na+ attract the negative ends of the H2O molecules, as shown in the diagram below. The ordered structure within the primary shell creates, through hydrogen-bonding, a region in which the surrounding waters are also somewhat ordered; this is the outer hydration shell, or cybotactic region.
Some recent experiments have revealed a degree of covalent bonding between the d-orbitals of transition metal ions and the oxygen atoms of water molecules in the inner hydration shell.
In 2003, some chemists in India found pp 816 – 818) that a suitable molecular backbone can cause water molecules to form a “thread” that can snake its way though the more open space of the larger molecules. What all of these examples show is that water can have highly organized local structures when it interacts with molecules capable of imposing these structures on the water.
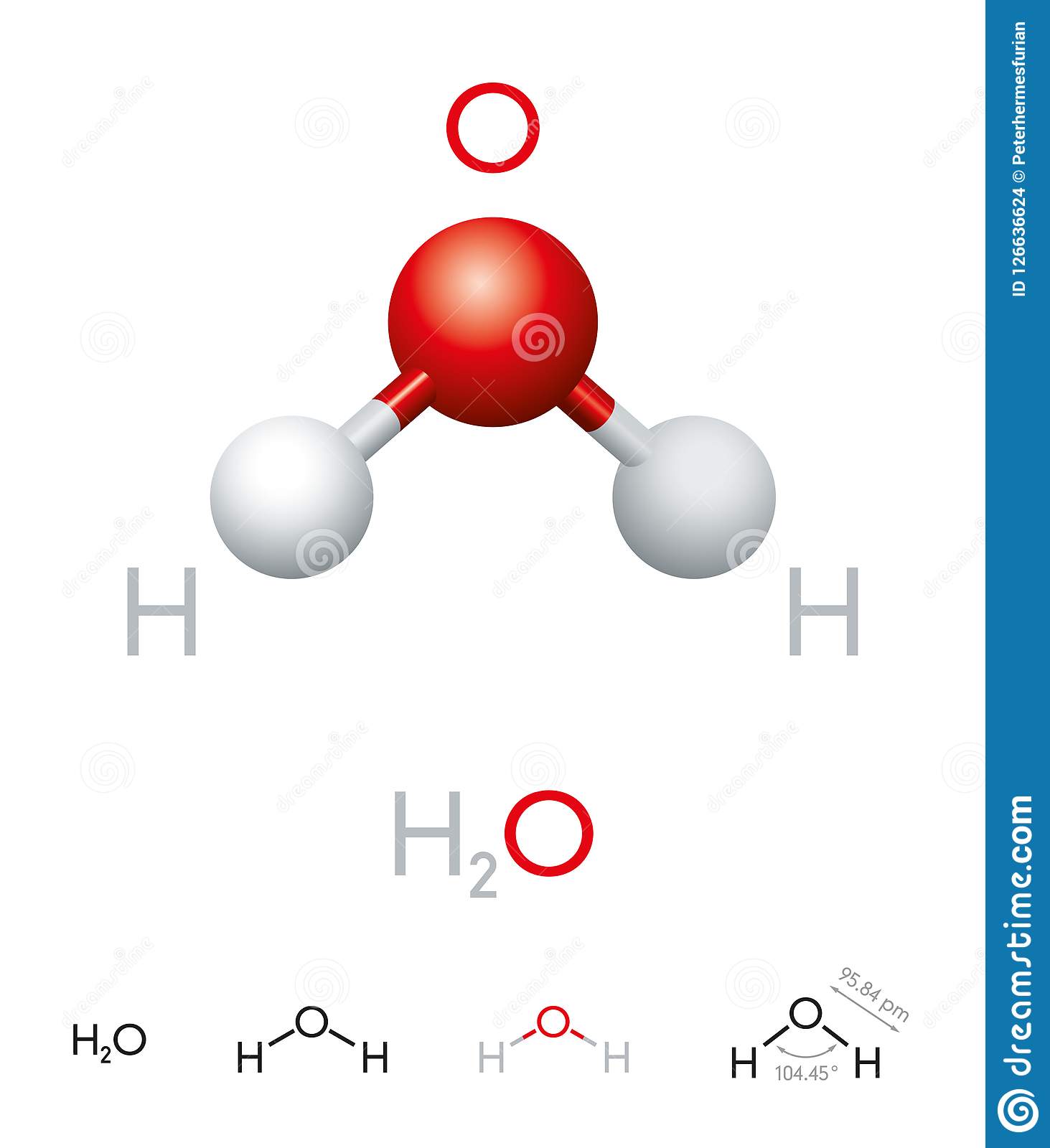
The Molecule Of Water
A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties and there are few molecules that are more stable and difficult to decompose than H2O. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; chemists call this shared electron pair a covalent chemical bond. In H2O, only two of the six outer-shell electrons of oxygen are used for this purpose, leaving four electrons which are organized into two non-bonding pairs. The four electron pairs surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsions between these clouds of negative charge. This would ordinarily result in a tetrahedral geometry in which the angle between electron pairs is 109.5°. However, because the two non-bonding pairs remain closer to the oxygen atom, these exert a stronger repulsion against the two covalent bonding pairs, effectively pushing the two hydrogen atoms closer together. The result is a distorted tetrahedral arrangement in which the HOH angle is 104.5°.
Although the water molecule carries no net electric charge, its eight electrons are not distributed uniformly; there is slightly more negative charge at the oxygen end of the molecule, and a compensating positive charge at the hydrogen end. The resulting polarity is largely responsible for water’s unique properties.
Read Also: Math Caching
The Structure And Properties Of Water
- Describe the structure and properties of water.
Key Points
- Water is a liquid at standard temperature and pressure .
- Water is is tasteless and odorless.
- Water is transparent in the visible part of the electromagnetic spectrum.
- Water can act as either an acid or a base.
- Water is a universal solvent, dissolving many substances found in nature.
Terms
- phase diagramA graph showing the phase a sample of matter has under different conditions of temperature and pressure.
- equilibriumThe state of a reaction in which the rates of the forward and reverse reactions are equal.
- dipoleAny molecule or radical that has delocalized positive and negative charges.
- amphotericA molecule that can act as either an acid or a base depending on its chemical environment. For example, water is amphoteric.
Why Does Ice Float On Water
When substances freeze, usually the molecules come closer together. Water has an abnormality there: it freezes below 0oC, but when temperatures goes below 4oC, water starts to expand again and as a result the density becomes lower. Density of a substance means the weight in kilograms of a cubic metre of a substance. When two substances are mixed but do not dissolve in one another, the substance with the lowest density floats on the other substance. In this case that substance is ice, due to the decreased density of water.
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
What Kind Of Water Is Most Healthy To Drink
I am not aware of any evidence indicating that any one type of water is more beneficial to health than any other, as long as the water is pathogen-free and meets accepted standards such as those mentioned above. For those who are sensitive to residual chlorine or still have concerns, a good activated-carbon filter is usually satisfactory. More extreme measures such as reverse-osmosis or distillation are only justified in demonstrably extreme situations.
“Pure” rainwater always contains some dissolved carbon dioxide which makes it slightly acidic. When this water comes into contact with sediments, it tends to dissolve them, and in the process becomes alkaline. The pH of drinking water can vary from around 5 to 9, and it has no effect on one’s health. The idea that alkaline water is better to drink than acidic water is widely promoted by alternative-health hucksters who market worthless “water ionizer” machines for this purpose. Acidic water is sometimes described by engineers as “aggressive”; this refers to its tendency to corrode metal distribution pipes, but in this sense it is no more active than the hydrochloric acid already present in your gastric fluid!
Electrical Conductivity And Electrolysis

Pure water has a low electrical conductivity, which increases with the dissolution of a small amount of ionic material such as common salt.
Liquid water can be split into the elements hydrogen and oxygen by passing an electric current through ita process called electrolysis. The decomposition requires more energy input than the heat released by the inverse process ” rel=”nofollow”>mol, or 15.9 MJ/kg).
Also Check: Geometry Segment Addition Postulate Worksheet Answer Key
Which Physical And Chemical Properties Does Water Have
There are several different physical and chemical properties, which are often used alternately. We can name the following: – Density. The density of water means the weight of a certain amount of water. It is usually expressed in kilograms per cubic metre. – Thermal properties. This refers to what happens to water when it is heated; at which temperature it becomes gaseous and that sort of thing. – Conductivity. This means the amount of electricity that water can conduct. It is expressed in a chemical magnitude. – Light absorption. This is the amount of light a certain amount of water can absorb over time. – Viscosity. This means the syrupiness of water and it determines the mobility of water. When the temperature rises, the viscosity degrades; this means that water will be more mobile at higher temperatures. – The pH. The pH has its own scale, running up from 1 to 14. The pH shows whether a substance is acid , neutral or basic . The number of hydrogen atoms in the substance determines the pH. The more hydrogen atoms a substance contains, the lower the pH will be. A substance that contains many hydrogen atoms is acid. We can measure the pH by dipping a special colouring paper in the substance, the colours shows which pH the substance has. – Alkalinity. This is the capacity of water to neutralize an acid or a base, so that the pH of the water will not change.
For water terminology check out our Water Glossary or go back to water FAQ overview
Interesting And Important Chemicals
Hydrates are interesting chemicals that are often very useful. Exploring their nature, properties, and behaviour is important. Gas hydrates are particularly interesting and are attracting the attention of many researchers. They could become very important in our future. There is much to learn about the best ways to use them and about safety procedures, however. Hopefully, their effects on our lives will be beneficial instead of harmful.
Also Check: Definition Of Abiotic In Biology
What Do Chemists Do
Chemists work in a variety of fields, including research and development, quality control, manufacturing, environmental protection, consulting and law. They can work at universities, for the government or in private industry, according to the ACS.
Here are some examples of what chemists do:
Research and development
In academia, chemists performing research aim to further knowledge about a particular topic, and may not necessarily have a specific application in mind. Their results, however, can still be applied to relevant products and applications.
In industry, chemists in research and development use scientific knowledge to develop or improve a specific product or process. For example, food chemists improve the quality, safety, storage and taste of food; pharmaceutical chemists develop and analyze the quality of drugs and other medical formulations; and agricultural chemists develop fertilizers, insecticides and herbicides necessary for large-scale crop production.
Sometimes, research and development may not involve bettering the product itself, but rather the manufacturing process involved in making that product. Chemical engineers and process engineers devise new ways to make the manufacturing of their products easier and more cost effective, such as increasing the speed and/or yield of a product for a given budget.
Environmental protection
Additional resources:;
Biowater: Bound Water In Biological Systems
It has long been known that the intracellular water very close to any membrane or organelle is organized very differently from bulk water, and that this structured water plays a significant role in governing the shape of large folded biopolymers. It is important to bear in mind, however, that the structure of the water in these regions is imposed solely by the geometry of the surrounding hydrogen bonding sites.
Water can hydrogen-bond not only to itself, but also to any other molecules that have -OH or -NH2 units hanging off of them. This includes simple molecules such as alcohols, surfaces such as glass, and macromolecules such as proteins. The biological activity of proteins is critically dependent not only on their composition but also on the way these huge molecules are folded; this folding involves hydrogen-bonded interactions with water, and also between different parts of the molecule itself. Anything that disrupts these intramolecular hydrogen bonds will denature the protein and destroy its biological activity. This is essentially what happens when you boil an egg; the bonds that hold the eggwhite protein in its compact folded arrangement break apart so that the molecules unfold into a tangled, insoluble mass which, like Humpty Dumpty, cannot be restored to their original forms. Note that hydrogen-bonding need not always involve water; thus the two parts of the DNA double helix are held together by HNH hydrogen bonds.
Also Check: What Is Copulation In Biology
Phase Diagram Of Water
Water freezes to form ice, ice thaws to form liquid water, and both water and ice can transform into the vapor state. Phase diagrams help describe how water changes states depending on the pressure and temperature.
Phase diagram of water
Note the following key points on a phase diagram:
- The critical point , above which only supercritical fluids exist.
- The triple point , a well-defined coordinate where the curves intersect, at which the three states of matter exist at equilibrium with each other.
- Well-defined boundaries between solid and liquid, solid and gas, and liquid and gas. During the phase transition between two phases , the phases are in equilibrium with each other.
Current Views Of Water Structure
The present thinking, influenced greatly by molecular modeling simulations beginning in the 1980s, is that on a very short time scale , water is more like a “gel” consisting of a single, huge hydrogen-bonded cluster. On a 10-12-10-9 sec time scale, rotations and other thermal motions cause individual hydrogen bonds to break and re-form in new configurations, inducing ever-changing local discontinuities whose extent and influence depends on the temperature and pressure.
|
Recent work from Richard SayKally’s lab shows that the hydrogen bonds in liquid water break and re-form so rapidly that the liquid can be regarded as a continuous network of hydrogen-bonded molecules. |
This computer-generated nanoscale view of liquid water is from the lab of Gene Stanley of Boston University. The oxygen atoms are red, the hydrogen atoms white |
You May Like: Unit 1 Study Guide Geometry Basics Answer Key
Classification Of Bacteria Based On Oxygen Requirement:
Pathogenic bacteriaMethods to measure the presence of coliform bacteria:
Coliform index
Surface Tension And Wetting
Have you ever watched an insect walk across the surface of a pond? The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. This is all due to the surface tension of the water. A molecule within the bulk of a liquid experiences attractions to neighboring molecules in all directions, but since these average out to zero, there is no net force on the molecule. For a molecule that finds itself at the surface, the situation is quite different; it experiences forces only sideways and downward, and this is what creates the stretched-membrane effect.
The distinction between molecules located at the surface and those deep inside is especially prominent in H2O, owing to the strong hydrogen-bonding forces. The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid’s surface tension.
This drawing highlights two H2O molecules, one at the surface, and the other in the bulk of the liquid. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in the 180° solid angle angle above the surface. As a consequence, a molecule at the surface will tend to be drawn into the bulk of the liquid. But since there must always be some surface, the overall effect is to minimize the surface area of a liquid.
Recommended Reading: What Math Class Do 11th Graders Take