Volume And Temperature: Charless Law
If we fill a balloon with air and seal it, the balloon contains a specific amount of air at atmospheric pressure, lets say 1 atm. If we put the balloon in a refrigerator, the gas inside gets cold and the balloon shrinks . If we make the balloon very cold, it will shrink a great deal, and it expands again when it warms up.
This video shows how cooling and heating a gas causes its volume to decrease or increase, respectively.
These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: The volume increases as the temperature increases, and decreases as the temperature decreases. Volume-temperature data for a 1-mole sample of methane gas at 1 atm are listed and graphed in Figure 4.
Figure 4.
The relationship between the volume and temperature of a given amount of gas at constant pressure is known as Charless law in recognition of the French scientist and balloon flight pioneer Jacques Alexandre César Charles. Charless law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant.
Mathematically, this can be written as:
with k being a proportionality constant that depends on the amount and pressure of the gas.
For a confined, constant pressure gas sample, \frac is constant , and as seen with the PT relationship, this leads to another form of Charless law: \frac = \frac.
Moles Of Gas And Volume: Avogadros Law
The Italian scientist Amedeo Avogadro advanced a hypothesis in 1811 to account for the behavior of gases, stating that equal volumes of all gases, measured under the same conditions of temperature and pressure, contain the same number of molecules. Over time, this relationship was supported by many experimental observations as expressed by Avogadros law: For a confined gas, the volume and number of moles are directly proportional if the pressure and temperature both remain constant.
In equation form, this is written as:
Mathematical relationships can also be determined for the other variable pairs, such as P versus n, and n versus T.
Visit this interactive PhET simulation to investigate the relationships between pressure, volume, temperature, and amount of gas. Use the simulation to examine the effect of changing one parameter on another while holding the other parameters constant .
How To Calculate In Standard Temperature And Pressure
Standard temperature and pressure calculations are not as hard as they seem – just follow our example below!
- Our volume = 5 L
- Our temperature = 350 K and
- Our pressure = 850 Torr.
VSTP = V * *
Also Check: Is Paris Jackson Really Michaels Daughter
What Are The Conditions For Stp In Chemistry
4.9/5temperaturetemperature
In respect to this, is STP 25 or 0?
Both STP and standard state conditions are commonly used for scientific calculations. STP stands for Standard Temperature and Pressure. It is defined to be 273 K and 1 atm pressure . Temperature is not specified, although most tables compile data at 25 degrees C .
Subsequently, question is, what does STP stand for? Standard Temperature and Pressure
In this way, what is the standard value for pressure at STP condition?
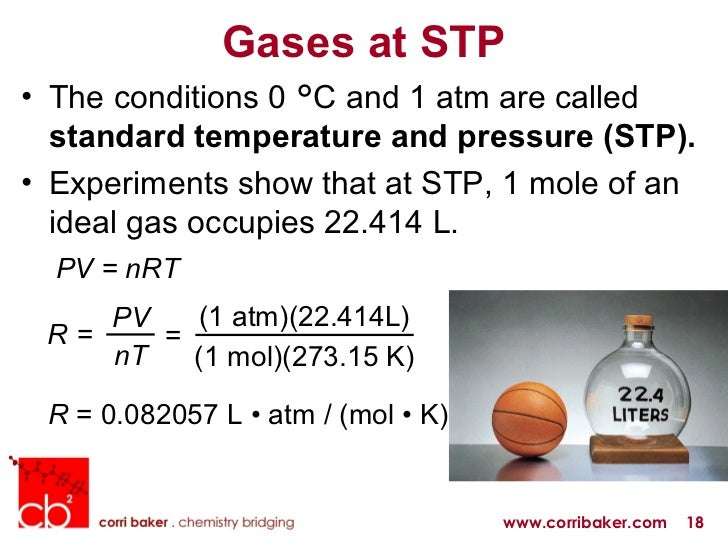
Standard Temperature and Pressure. Standard temperature is equal to 0 °C, which is 273.15 K. Standard Pressure is 1 Atm, 101.3kPa or 760 mmHg or torr. STP is the “standard” conditions often used for measuring gas density and volume.
What is PV nRT called?
PV = nRT: The Ideal Gas Law. Fifteen ExamplesEach unit occurs three times and the cube root yields L-atm / mol-K, the correct units for R when used in a gas law context. Consequently, we have: PV / nT = R. or, more commonly: PV = nRT. R is the gas constant.
Chemistry End Of Chapter Exercises
the appropriate graph
Boyles law
Read Also: Mark Lester Paris Jackson
Molar Volume Of A Gas
It is equally as important to indicate the applicable reference conditions of temperature and pressure when stating the molar volume of a gas as it is when expressing a gas volume or volumetric flow rate. Stating the molar volume of a gas without indicating the reference conditions of temperature and pressure has very little meaning and can cause confusion.
The molar volume of gases around STP and at atmospheric pressure can be calculated with an accuracy that is usually sufficient by using the ideal gas law. The molar volume of any ideal gas may be calculated at various standard reference conditions as shown below:
- Vm = 8.3145 × 273.15 / 101.325 = 22.414 dm3/mol at 0 °C and 101.325 kPa
- Vm = 8.3145 × 273.15 / 100.000 = 22.711 dm3/mol at 0 °C and 100 kPa
- Vm = 8.3145 × 298.15 / 101.325 = 24.466 dm3/mol at 25 °C and 101.325 kPa
- Vm = 8.3145 × 298.15 / 100.000 = 24.790 dm3/mol at 25 °C and 100 kPa
- Vm = 10.7316 × 519.67 / 14.696 = 379.48 ft3/lbmol at 60 °F and 14.696 psi
- Vm = 10.7316 × 519.67 / 14.730 = 378.61 ft3/lbmol at 60 °F and 14.73 psi
Technical literature can be confusing because many authors fail to explain whether they are using the ideal gas constantR, or the specific gas constant Rs. The relationship between the two constants is Rs = R / m, where m is the molecular mass of the gas.
Standard Temperature And Pressure
Standard temperature and pressure are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union of Pure and Applied Chemistry and the National Institute of Standards and Technology , although these are not universally accepted standards. Other organizations have established a variety of alternative definitions for their standard reference conditions.
In chemistry, IUPAC changed its definition of standard temperature and pressure in 1982:
- Until 1982, STP was defined as a temperature of 273.15 K and an absolute pressure of exactly 1 atm ” rel=”nofollow”> kPa).
- Since 1982, STP has been defined as a temperature of 273.15 K and an absolute pressure of exactly 105 Pa ” rel=”nofollow”> bar).
STP should not be confused with the standard state commonly used in thermodynamic evaluations of the Gibbs energy of a reaction.
NIST uses a temperature of 20 °C and an absolute pressure of 1 atm . This standard is also called normal temperature and pressure . However, a common temperature and pressure in use by NIST for thermodynamic experiments is 298.15 K and 1 bar ” rel=”nofollow”> kPa). NIST also uses “15 °C ” for the temperature compensation of refined petroleum products, despite noting that these two values are not exactly consistent with each other.
Read Also: Kw Meaning Chemistry
Supercritical Carbon Dioxide Extraction
Carbon dioxide usually behaves as a gas in air at standard temperature and pressure or as a solid called dry ice when frozen . If the temperature and pressure are both increased from STP above the critical point for CO2, it can adopt properties midway between a gas and a liquid and behave as a supercritical fluid, thus expanding like a gas but with a density like that of a liquid. Supercritical CO2 is becoming an important commercial and industrial solvent due to its role in chemical extraction in addition to its low toxicity and environmental impact . The relatively low temperature of the process and the stability of CO2 also allow most compounds especially in food industry to be extracted with little damage or denaturing. The main drawbacks of this method include high power consumption, expensive and difficulty involved in scaling up .
Md. Saiful Alam, Md. Sifat Tanveer, in, 2020
Key Takeaways: Stp Or Standard Temperature And Pressure
- STP is the abbreviation for Standard Temperature and Pressure. However, the “standard” is defined differently by various groups.
- STP values are most often cited for gases because their characteristics change dramatically with temperature and pressure.
- One common definition of STP is a temperature of 273 K and the standard pressure of 1 atm. Under these conditions, one mole of a gas occupies 22.4 L.
- Because the standard varies by industry, it’s good practice to state temperature and pressure conditions for measurements and not just say “STP.”
Recommended Reading: Definition For Movement In Geography
Why Do We Need Stp
Let say a chemist in a laboratory is performing a chemical experiment in London. Mean room temperature and pressure in London is 12 and 1.015 bar. If the same experiment is conducted by him/her in Austin, where mean room temperature and pressure is 21 and 1.014 bar, the result of the experiments might significantly vary. This is because many scientific experiments, particularly of chemistry, are influenced by temperature and pressure. Hence, to avoid such deviations we have adopted some standard conditions so that experiments all over the world are performed in similar laboratory conditions. One of such standards is STP .
Standard Conditions Of Temperature And Pressure
We have seen that the volume of a given quantity of gas and the number of molecules in a given volume of gas vary with changes in pressure and temperature. Chemists sometimes make comparisons against a standard temperature and pressure for reporting properties of gases: 273.15 K and 1 atm . At STP, an ideal gas has a volume of about 22.4 Lthis is referred to as the standard molar volume .
Figure 10.
Recommended Reading: Beth Thomas
Key Concepts And Summary
The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change . The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure . The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant . Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules .
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal gas constant.
Discover And Learn What Students Are Asking
?Think About It The point lies on the graph of f, and the slope of the tangent line through this point is m = 2. Assume f-1 exists. What is the ?In Exercises 712, use sigma notation to write the sum.\?In Exercises 1-4, write an integral that represents the area of the shaded region of the figure. Do not evaluate the integral.\\) and \\). These two substances react as follows:\ Is \ the same in magnitude for both the forward and reverse proces?Bear Markets A bear market in the stock market is defined as a condition in which the market declines by 20% or more over the course of at least two m?In Problems 29 and 30, draw a normal curve and label the mean and inflection points.? = 30 and ? = 10
Recommended Reading: Scientific Definition Of Abiotic
What Is The Difference Between Standard Temperature And Pressure And Standard State
Standard temperature and pressure defined to be 0 degrees Celsius and 1 atm pressure describes standard conditions and is used to measure gas density and volume using the Ideal Gas Law. Meanwhile, standard state conditions are employed for thermodynamic calculations. Both the standard state and STP specify a gas pressure of 1 atmosphere, however, the standard temperature in the standard state doesnt remain constant it keeps changing.
Was this answer helpful?
Why Do We Need Standards
Chemists require STP definitions because the behavior of a substance varies greatly depending on the temperature and pressure. STP definitions give chemists a common reference point to describe how a gas behaves under normal conditions. Scientists use standards like STP definitions for two purposes, to define certain quantitative metrics and to allow for consistent and repeatable experiments.
Imagine someone tells you the molar volume of methane is 22.4 liters . The molar volume of a substance is just a measure of how much space one mole of that substance takes up. On its own, this value is not very informative. It is known that the volume of a gas varies greatly with respect to pressure and temperature, so gas could have multiple molar volumes, depending on the exact temperature and pressure. One needs to specify a temperature and pressure to make a molar volume measurement of 22.4 L a more meaningful quantity. Scientists agree upon a predefined temperature and pressure to report quantitative properties of gases. As it just so happens, one mole of any gas at STP has a volume of 22.4 L. Quantitative measurements of gas, like volume, volumetric flow, and compressibility, all must be defined with respect to some defined pressure and temperature.
The true method of knowledge is experiment. William Blake
Don’t Miss: Homework 2 Segment Addition Postulate
Definitions Used In The Past
For a great many years, most engineers, chemists, physicists and other scientists using the metric system of units defined the standard reference conditions of temperature and pressure for expressing gas volumes as being 0 °C and 101.325 kPa . During those same years, the most commonly used standard reference conditions for people using the Imperial or customary USA system of units was 60 °F and 14.696 psia because it was almost universally used by the oil and gas industries worldwide.
The above two definitions are no longer the most commonly used definitions in either the metric, Imperial or the customary USA system of units. Some of the many different definitions currently in use are presented in the next section.
It was also common in the past, when using the metric system of units, to refer to a Normal Cubic Meter and to define it as being at 0 °C and 101.325 kPa . As shown in the following section, that notation is no longer appropriate unless the specific reference conditions are explicitly stated, since there are currently many different metric system definitions of what constitutes standard reference conditions.
How To Use Our Standard Temperature And Pressure Calculator
Our standard temperature and pressure calculator requires four easy steps:
Remember, our calculators work both ways! Whatever it is you’re trying to calculate, we’re here for you.
Read Also: Age Word Problems Worksheet
Definitions Of Standard Temperature And Pressure
The term STP means different things to different people and can cause problems in the presentation of adsorption data, because the most common units for the ordinates of such plots are standard volumes per unit mass of adsorbent.
The International Union of Pure and Applied Chemistry used to define STP as 0 C and 1 atm . This definition is now obsolete. The preferred definition, since 1982, is 273.15 K and 1 bar . The National Institute of Standards and Technology, on the other hand, defines STP as 1 atm and 20 C . In practical applications of adsorption equipment, STP often refers to the pressure of the room and the temperature of the adsorption manifold, which is often kept above room temperature to prevent changes in room temperature from changing the apparent manifold or dead volumes . Room temperature, at least in the summer months in many places, is usually close to 25 C, which is a de facto standard in gas flow controllers.
For the sake of comparison, we recommend specifying the definitions of STP being used if there is any concern that they will affect the results. The difference between using a standard temperature of 0 C and 25 C will, in the authors’ experience, produce errors in the volume adsorbed that are less than the error in the measurements themselves. In commercial equipment, the volume adsorbed is usually reported by defining STP as 0 C and 760 Torr .
Alain Tressaud, in, 2019