Identification Of Polar And Nonpolar Bonds
As mentioned earlier, there could be the possibilities of two types of bonds, either it could be completely polar or nonpolar. When there is no disparity between the electronegativities of molecules, the bond will be nonpolar covalent bonds. On the other hand, when the more electronegative atom pulls an electron from the other atom, then polar ionic bonds will be formed.
Bond identification is represented in a tabular format below in terms of electronegativity:
|
Type of bond |
|
|
Ionic |
> 1.8 |
The difference in Electronegativity is the major reason due to the difference between polar and nonpolar bonds.
Title: Cool Molecular Highly Charged Ions For Precision Tests Of Fundamental Physics
Abstract: Molecules and atomic highly charged ions provide powerful low-energy probesof the fundamental laws of physics: Polar molecules possess internal fieldssuitable to enhance fundamental symmetry violation by several orders ofmagnitudes, whereas atoms in high charge states can feature large relativisticeffects and compressed level structures, ideally posed for high sensitivity tovariations of fundamental constants. Polar, highly charged molecules couldbenefit from both: large internal fields and large relativistic effects.However, a high charge dramatically weakens chemical bonding and drives systemsto the edge of Coulomb explosion. Herein, we propose multiply-charged polarmolecules, that contain actinides, as promising candidates for precision testsof physics beyond the standard model. Explicitly, we predict PaF$^$ to bethermodynamically stable, coolable and well-suited for precision spectroscopy.The proposed class of compounds, especially with short-lived actinide isotopesfrom the territory of pear-shaped nuclei, has potential to advance ourunderstanding of molecules under extreme conditions, to provide a window intounknown properties of atomic nuclei, and to boost developments in molecularprecision spectroscopy in various areas, such as optical clocks and searchesfor new physics.
Polar Molecule: Definition And Examples
Alex BolanoPRO INVESTOR
Polarity in chemistry refers to the unequal attraction of electrons in elements of a compound, resulting in a molecule with a negatively charged end and a positively charged end. The polarity of a molecule depends upon the electronegativity of the constituent elements of that molecule.
Elements that differ greatly in electronegativities will exert unequal attractions on electrons, creating a net difference in charge over the molecule. The polarity of individual bonds also determines a molecules shape as the unequal pull of electrons affects the spatial orientation of the molecules.
Polarity, or action and reaction, we meet in every part of nature in darkness and light in heat and cold in the ebb and flow of water in male and female in the equation of quantity and quality in the fluids of the animal body. Ralph Waldo Emerson
Polarity is an important concept as it determines the number of physical properties of a substance. The polarity of a substance determines its surface tension, solubility, and melting/boiling point. Polar molecules interact in characteristic ways via hydrogen bonding and dipole-dipole interactions.
Read Also: Lewis Structure Of Ccl4
Polar Versus Nonpolar Molecular Geometry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The two main classes of molecules are polar molecules and nonpolar molecules. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. Here’s a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds.
What Are Polar Solvents
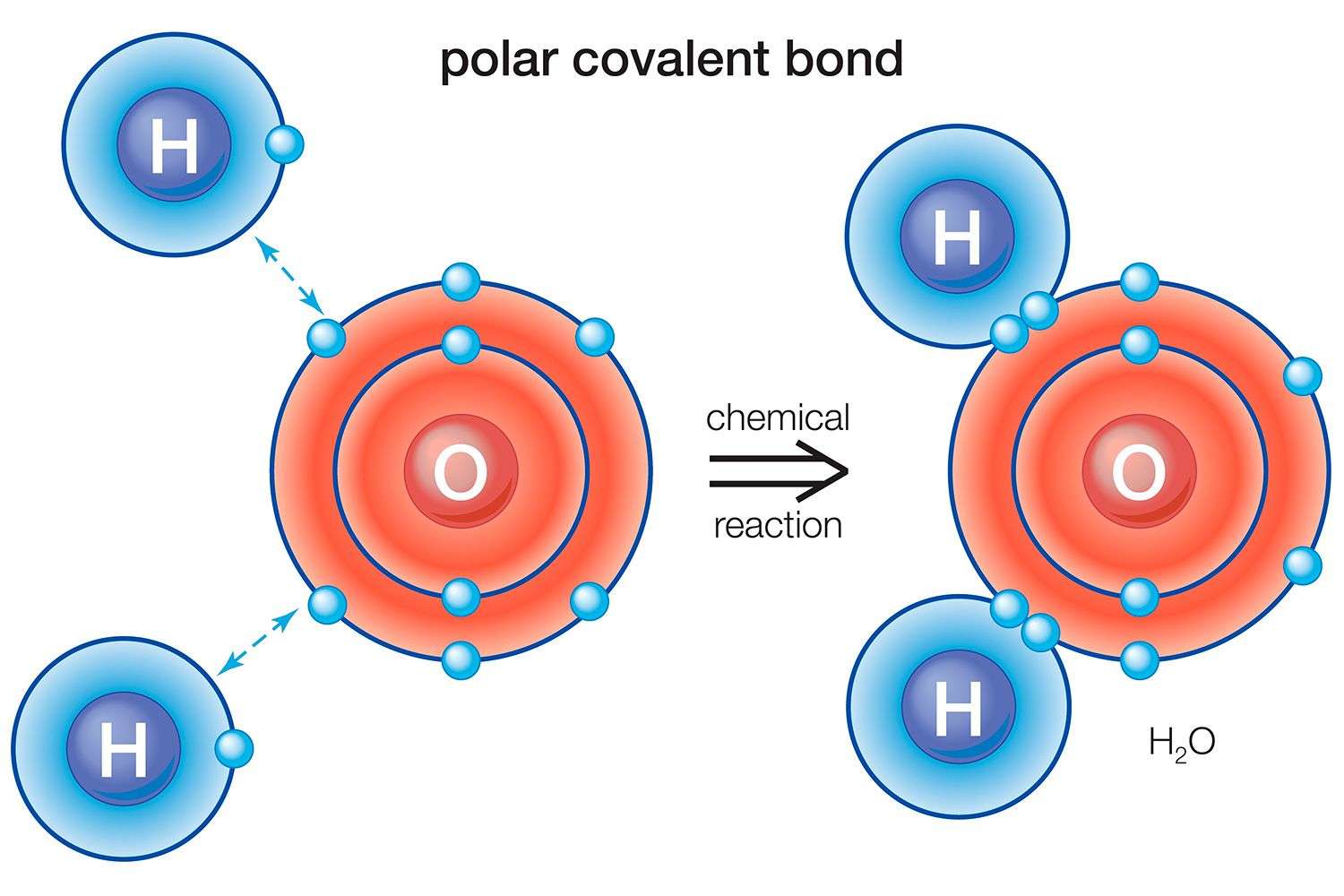
Generally, polar molecules and polar solvents possess large dipole moment values. Polar solvents are liquids that can dissolve various polar compounds. This is because the positively charged molecule of a compound gets easily attracted by the negatively charged molecule of a solvent, which leads to the liquefaction of the polar compounds to the polar solvents. It is previously illustrated that the polarity of the solvent arises because of disparity in the electronegativity of molecules.
Recommended Reading: How Was Ancient Greek Civilization And Culture Affected By Geography
Electron Capture By Polar Molecules
Jean-Marc Lévy-Leblond
Abstract
It is shown that binding of electrons in the electric dipole field of polar molecules cannot possibly occur, if the molecules have an electric dipole moment smaller than the critical value D 18 esu cm, irrespective of the size of the dipole. This result casts doubts on a proposed mechanism of electron scattering by polar molecules, namely, electron capture with rotational excitation of the molecule.
- Received 15 August 1966
- Department of Physics and Astronomy, University of Rochester, Rochester, New York
- *Permanent address: Physique Théorique Faculté des Sciences 06-Nice, France.
ISSN 1536-6065 . ©2022 American Physical Society. All rights reserved. Physical Review, Physical Review Letters, Physical Review X, Reviews of Modern Physics, Physical Review A, Physical Review B, Physical Review C, Physical Review D, Physical Review E, Physical Review Applied, Physical Review Fluids, Physical Review Accelerators and Beams, Physical Review Physics Education Research, APS Physics logo, and Physics logo are trademarks of the American Physical Society. Information about registration may be found here. Use of the American Physical Society websites and journals implies that the user has read and agrees to our Terms and Conditions and any applicableSubscription Agreement.
Log In
Instances Of Polar Molecules
Carbon dioxide is comprised of polar bonds. However, the dipole minutes offset one another and along these lines is certifiably not a polar atom.
To decide a particles extremity, first, ensure you have an intermittent table of components convenient . Decide every molecules electronegativity from the table. You can decide a non-polar particle promptly if the iotas in the atom have equivalent electronegativity.
A particle can likewise be non-polar if the structure of the molecules line up so that the external orbital electrons counterbalance electronegativity. In this way, you have to look at the state of the particle. By and large, V-formed and pyramid-moulded atoms are polar. Straight particles will, in general, be non-polar.
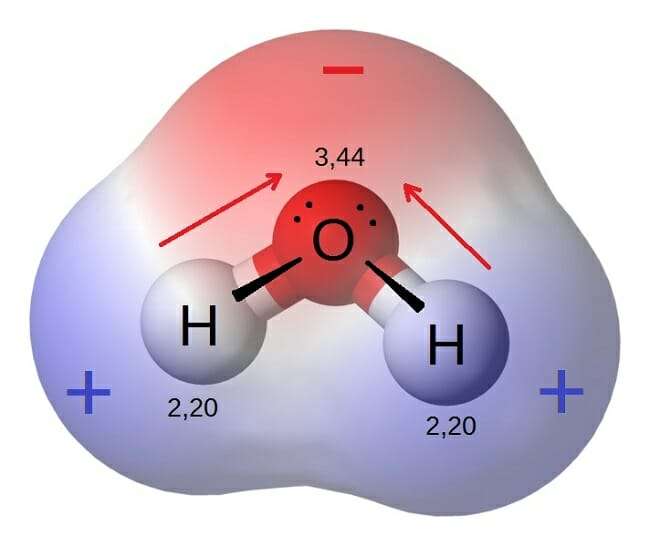
Water is polar as a result of the distinctions in the electronegativity among oxygen and hydrogen. Oxygen is profoundly negative in contrast with hydrogen.
The oxygen pulls in negative charges, making the region around the oxygen significantly more harmful than the region around the hydrogen. The hydrogen region makes the particle twist with the goal that the two hydrogen molecules in the water are on a similar side, pointing ceaselessly from the negative territory around the oxygen.
Glucose is another case of a polar particle as a result of how the oxygen iotas and hydrogen molecules are organised. Glucose has a hexagon shape made out of 6 carbon molecules, 12 hydrogen particles, and six oxygen iotas.
Foreseeing Polarity and Nonpolarity
You May Like: Ct Algebra 1 Curriculum Version 3.0
What Is A Polar Molecule
A polar molecule has one side that has a positive charge density and another side that has a negative charge density.
Explanation:
# CCl_4# is a non polar molecule because even thought each bond is highly polar the bondrs are symmetrical arranged and so all four sides of the molecule are negative.
# NH_3# is polar. The bonds between the N and the H are not highly polar but there is a positive density on all of the Hydrogens The unbonded pair of electrons on the nitrogen have a negative density. The structure of the molecule is such that the three bonded Hydrogen are in the same plane while the unbounded electrons are in a different plane than the Hydrogens this creates a non polar molecule.
Polarity And Mixing Solutions
If you know the polarity of molecules, you can predict whether or not they will mix together to form chemical solutions. The general rule is that “like dissolves like”, which means polar molecules will dissolve into other polar liquids and nonpolar molecules will dissolve into nonpolar liquids. This is why oil and water don’t mix: oil is nonpolar while water is polar.
It’s helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldn’t mix with otherwise. For example, if you want to mix an ionic compound or polar compound in an organic solvent, you may be able to dissolve it in ethanol . Then, you can dissolve the ethanol solution into an organic solvent, such as xylene.
Read Also: New England Climate And Geography
Insulators And Dielectric Material
Insulators are materials with very poor conductivity. Insulators are not very good at conducting either heat or electricity owing to the absence of loosely bound or freely moving charges in the atoms of the insulator. When insulators are placed in an electric field, practically no current flows in them, unlike in metals. Instead, in some insulators, electric polarization occurs. A dielectric is an insulator that undergoes electric polarization on the application of an electric field. The charges in dielectric material do not move but only shift slightly from the equilibrium position resulting in the dielectric polarization. Not every insulator is a dielectric material, the necessary condition for dielectric polarization is the presence of polar molecules. Lets explore this concept.
What Makes A Bond Polar
Polar bonds are a type of covalent bond. Polar bonds are formed when two or more atoms have significantly different electronegativities . These bonds do not share electrons equally, which means the negative charge from electrons is not evenly distributed in the molecule. This causes a dipole moment. If there is one positive end of the bond and one negative end of the bond, then a dipole moment has occurred. Historically, polar bonds have been found in the bonds between hydrogen and oxygen in water. As a result of the electrodepositivity difference of 1, the bond is referred to as a polar bond. Hydrogen electrons are more attracted to oxygen electrons because oxygen is more electronegative.
You May Like: How Do You Find Displacement
What Are Polar Compounds
The chemical compounds that are held together by polar covalent bonds are known as polar compounds. The word ‘polar compound’ can be defined as a chemical species consisting of two or more atoms that are kept together due to the unequal sharing of electrons by covalent bonds that are polar in nature. The differences in the electronegativities of the bonded atoms may cause the bond pair of electrons to move closer to the more electronegative atom when two atoms are bound together by a covalent bond.
What Are Polar And Non Polar Molecules In Physics
Devansh Shukla answered this
Non Polar Molecules Are The Molecules In Which The Centre Of Positive Andd Negative Charge Co-incides.The Molecule Then Has No Dipole Moment.Ex:- H2, O2
Polar Molecules Are The Molecules In Which The Centre Of Positive And Negative Charges Are Seperated.Then Such Molecules Have Permanent Dipole Moment.Ex:- HCl , H2O
- 3
Manjeet Kaur answered this
in polar molecules the centre of positive nd negative charges do not coincide because of the asymmetrical shapes of the molecules.
in non polar molecules the centre of positive charges coincide with the centre of negative charges
- 0
Recommended Reading: How Many Daughters Does Steve Harvey Have
What Is The Difference Between Polar And Dipolar Molecules
The key difference between polar and dipolar molecules is that polar molecules have two opposite ends with opposite electrical charges, whereas dipolar molecules have two poles. However, in general terms, we can use the terms polar and dipolar interchangeably because both these terms describe a single molecule having two opposite ends.
Besides, another significant difference between polar and dipolar molecules is that polar molecules form when there is a charge separation while dipolar molecules form due to the difference in electronegativity values of atoms.
Below is a summary tabulation of the difference between polar and dipolar molecules.
Main Difference Polar Vs Nonpolar Molecules
Atoms of different or same elements come together to form molecules. The bond which is formed by sharing a pair of electrons between two atoms is called a Covalent Bond. Different atoms show attraction to electrons in various degrees. Their ability to pull electrons towards them is called electronegativity. Atoms such as F, Cl, O show greater electronegativity comparative to atoms like C, P, S. When two atoms with 0.4< electronegativity difference are bonded, polar molecules are formed. If the electronegativity difference between the atoms is < 0.4 the molecule becomes non-polar. The main difference between polar and nonpolar molecules is net dipole moment. The net dipole moment is formed on the atoms of polar molecules, but not on non-polar molecules.
This article explains,
1. What are Polar Molecules Definition, Formation, Properties, Examples
2. What are Nonpolar Molecules Definition, Formation, Properties, Examples
3. What is the difference between Polar and Nonpolar Molecules
Don’t Miss: Prentice Hall Gold Algebra 1 Teaching Resources Answers Chapter 9
Is Polarisation Is Temperature
· There is a large fluctuation in the dielectric constant with temperature for materials having permanent electric dipoles. The influence of temperature on orientational polarisation is the reason behind this.
· However, this does not imply that as the temperature is decreased, the dielectric constant will continue to rise. As the temperature fluctuates, there are various discontinuities in the dielectric constant. First and foremost, the dielectric constant will change abruptly at phase boundaries. As weve seen, the structure changes during a phase change and the dielectric constant is highly reliant on the structure. The exact two phases involved determine whether will increase or decrease at a specific phase transition.
· At a temperature below the freezing point, there is also a significant drop in .
· The potential energy of orientations aligned with the field is dropped when an electric field is applied, whereas the energy of orientation positioned against the field is raised. This means that switching between orientations aligned with the field requires less energy, whereas switching to orientations lined against the field requires more.
Adsorption Of Polar Molecules On A Nonpolar Adsorbent
In the adsorption of polar molecules on a nonpolar adsorbent the constant dipole moment of the adsorbate molecule polarizes the adsorbent atoms, i.e. induces an electric moment in them. This leads to an inductive attraction in addition to the dispersion attraction. Depending on the position and magnitude of the dipole in the adsorbate molecule, and the polarizability of the adsorbent, the induction force energy can attain values up to several kJ mol1. For example, in the adsorption of molecules with a dipole moment of 1 on graphite , the contribution of the induction forces to the overall potential energy of adsorption is:
Read Also: Calculate Half Life Formula
How Cold Are Ultracold Polar Molecules
Ultracold polar molecules have been created by research scientists. These “molecules” consist of \ and \ atoms excited by lasers to form a type of \ compound where the \ has a positive charge and the \ has a negative charge. The material is formed at temperatures extremely close to absolute zero. The researchers believe these techniques will help them make new reactions and new materials.
Why Is A Molecule Polar
Polar molecules result from differences in electronegativity of the atoms in the molecule. Dipoles that are directly opposite one another cancel each other out.
What is polar covalent and nonpolar covalent?
A covalent bond that has an unequal sharing of electrons, as in part of Figure 4.4. 1, is called a polar covalent bond. A covalent bond that has an equal sharing of electrons of Figure 4.4. 1) is called a nonpolar covalent bond.
You May Like: What Influence Did Geography Have On Greek Society
Examples Of Polar Molecules
- Water is a polar molecule. The bonds between hydrogen and oxygen are distributed so that the hydrogen atoms are both on one side of the oxygen atom rather than evenly spaced. The oxygen side of the molecule has a slight negative charge, while the side with the hydrogen atoms has a slight positive charge.
- Ethanol is polar because the oxygen atoms attract electrons because of their higher electronegativity than other atoms in the molecule. Thus the -OH group in ethanol has a slight negative charge.
- Ammonia is polar.
- Sulfur dioxide is polar.
- Hydrogen sulfide is polar.
Carbon dioxide is made up of polar bonds, but the dipole moments cancel each other out. Therefore it is not a polar molecule.
Difference Between Polar And Non
The prime difference between polar and nonpolar solvents is, the polar solvent gets dissolved in a polar compound, whereas the non-polar solvent gets dissolved in non-polar compounds. Well, moreover, the polar solvents possess molecules with polar bonds, and nonpolar solvents possess molecules with similar electronegativity values.
Recommended Reading: Kendall Hunt Geometry
Difference Between Polar And Nonpolar Compounds
To understand the difference between polar and nonpolar compounds, it is necessary to concentrate upon the Lewis structure. The non-polar compounds will be symmetric, which means the presence of identical atoms around the central atom, which bonds to the element without any unshared pairs of electrons. While taking into consideration the CCl4 molecule, it is completely non-polar due to its tetrahedral structure.
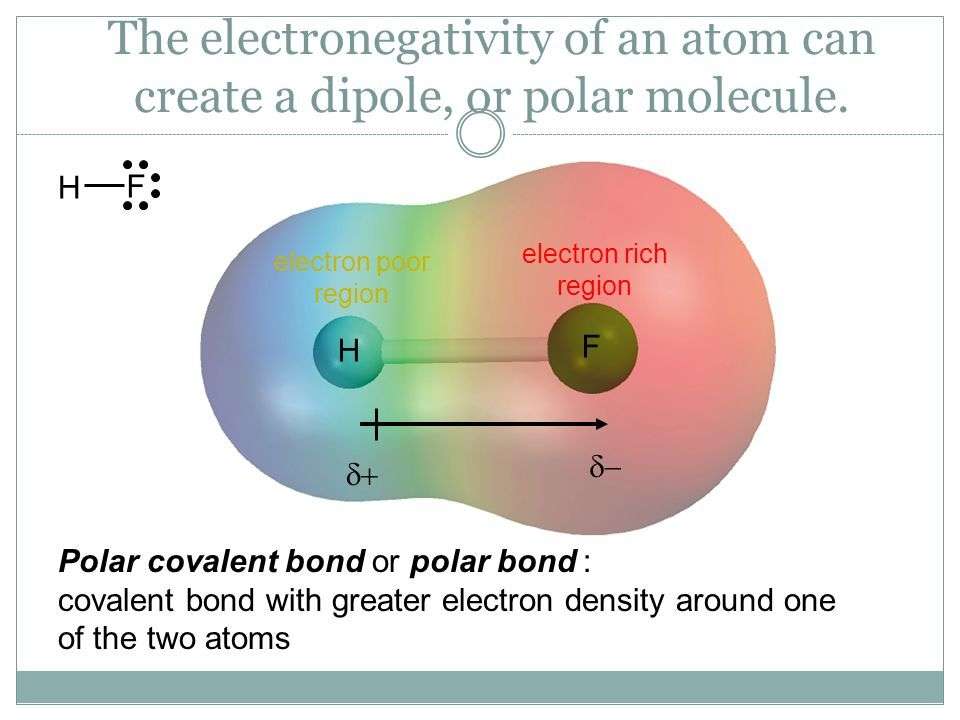
As compared to the non-polar compounds, polar compounds are asymmetric in nature as they contain lone pairs of electrons on a central atom, and the attached atoms possess different electronegativities. For example, Hydrogen Fluoride is a diatomic molecule, with one side being slightly positive, and another side being slightly negative. This disparity in electronegativity makes it a polar compound. The bond is a polar covalent bond.
The high electronegativity of the Fluorine atom drags all the positive charges from the H atom. This is why a partial positive charge has been generated on the H atom and a partial negative charge on the F atom. The entire molecule is considered a dipole molecule due to the unequal distribution of electron density.
Whereas water possesses a bent structure and due to the higher electronegativity of oxygen, it pulls out the charges and so that the direct will be H to O. Due to this structure, the dipoles cannot cancel out each other and the compound is polar.