Uninterruptible Power Supply Systems
Lithium-ion batteries are becoming increasingly more commonplace in Uninterruptible power supply systems. They offer numerous benefits over the traditional VRLA battery and with stability and safety improvements confidence in the technology is growing. Their power to size and weight ratio is seen as a major benefit in many industries requiring critical power back up including data centers where space is often at a premium. The longer cycle life, usable energy , and thermal runaway are also seen as a benefit for using Li-po batteries over VRLA batteries.
Why Lfp Is The Best Choice For Material Handling Operations
As stated above, LFP chemistry is the optimal choice for material handling equipment batteries, and heres why.
Of all the various types of lithium-ion batteries, three cell chemistry types emerge as widely used in on- and off-highway electric vehicles: lithium ferrophosphate, or lithium iron phosphate , lithium nickel manganese cobalt oxide , and lithium nickel cobalt aluminum oxide .
All batteries degrade with usage, decreasing their Ah capacity with each charge/discharge cycle. In material handling, batteries usually become unusable when they drop below 80% of their nominal capacity.
A batterys longevity, or its cycle life, depends on three main factors:
- chemical composition of cathode materials
- ambient temperature of operation
- depth of discharge.
The graph below shows the results of recent independent degradation tests of the three types of cells with different chemistry, under equal conditions of temperature and depth of discharge.
One equivalent full cycle is the sum of charge/discharge events that add up to one full charge and one full discharge of a battery.
LFP lithium batteries exhibit superior performance compared to NMCthey offer a longer lifespan and are generally less expensive.
Lithium Cells With Solid Polymer Electrolyte
Cells with solid polymer electrolytes have not reached full commercialization and are still a topic of research. Prototype cells of this type could be considered to be between a traditional lithium-ion battery and a completely plastic, solid-state lithium-ion battery.
The simplest approach is to use a polymer matrix, such as polyvinylidene fluoride or poly , gelled with conventional salts and solvents, such as LiPF6 in EC/DMC/.
Nishi mentions that Sony started research on lithium-ion cells with gelled polymer electrolytes in 1988, before the commercialisation of the liquid-electrolyte lithium-ion cell in 1991. At that time polymer batteries were promising and it seemed polymer electrolytes would become indispensable. Eventually, this type of cell went into the market in 1998.However, Scrosati argues that, in the strictest sense, gelled membranes cannot be classified as “true” polymer electrolytes, but rather as hybrid systems where the liquid phases are contained within the polymer matrix. Although these polymer electrolytes may be dry to the touch, they can still contain 30% to 50% liquid solvent. In this regard, how to really define what a “polymer battery” is remains an open question.
A solid polymer electrolyte is a solvent-free salt solution in a polymer medium. It may be, for example, a compound of lithium bisimide and high molecular weight poly , or a high molecular weight poly .
Also Check: Edgenuity Unit Test Answers
Glutamate And Nmda Receptors
Glutamate levels are observed to be elevated during mania. Lithium is thought to provide long-term mood stabilization and have anti-manic properties by modulating glutamate levels. It is proposed that lithium competes with magnesium for binding to NMDA glutamate receptor, increasing the availability of glutamate in post-synapticneurons.The NMDA receptor is also affected by other neurotransmitters such as serotonin and dopamine. Effects observed appear exclusive to lithium and have not been observed by other monovalent ions such as rubidium and caesium.
Diagonal Relationship With Magnesium
Lithium has a diagonal relationship with magnesium. Both elements have many similarities, such as they possess the same atomic and ionic radius, and both have almost similar electronegativities. Both elements form nitrides when they react with nitrogen, and they form oxides and peroxides when they react with oxygen.
Recommended Reading: Unit Test Edgenuity Answers
How Does Recharging A Lithium
When the lithium-ion battery in your mobile phone is powering it, positively charged lithium ions move from the negative anode to the positive cathode. They do this by moving through the electrolyte until they reach the positive electrode. There, they are deposited. The electrons, on the other hand, move from the anode to the cathode.
When the battery is in use, the lithium ions flow from the anode to the cathode, and the electrons move from the cathode to the anode.
When you charge a lithium-ion battery, the exact opposite process happens. The lithium ions move back from the cathode to the anode. The electrons move from the anode to the cathode.
Illustration – Text Version
When the battery is charging, the lithium ions flow from the cathode to the anode, and the electrons move from the anode to the cathode.
As long as lithium ions are making the trek from one electrode to another, there is a constant flow of electrons. This provides the energy to keep your device running. Since this cycle can be repeated hundreds of times, this type of battery is rechargeable.
Did you know?
Sometimes lithium-ion batteries are referred to as “rocking chair batteries.” This is because lithium ions ‘rock’ back and forth between the electrodes.
Are There Any Risks With Using Lithium
While these batteries are pretty impressive, they do have their downsides. The biggest complaint is that they wear out fairly quickly whether you use them or not. A typical lithium-ion battery will last about 2-3 years before it has to be replaced. That can get expensive! The production and disposal of lithium-ion batteries also has a big impact on the environment, so the longer those batteries can last the better.
As you learned, lithium is extremely reactive. When manufacturers make lithium-ion batteries, they have to take certain precautions so that the batteries are safe to use. However, you may have heard of some electronics, such as laptops or cell phones, bursting into flames because of their batteries. While this might be a good excuse for not handing in your English essay on time, its a pretty dangerous situation. For safety reasons, lithium-ion batteries include a . This prevents the electrodes of the batterys cells from touching each other. But if this separator gets ripped or damaged, the electrodes can touch. This can cause a huge build-up of heat. If this build-up of heat produces a spark, the highly flammable electrolyte can catch on fire.
Once there are flames in one cell, they can quickly spread to others. And before you know it, your laptop is a pool of melted plastic. A build-up of heat can also cause the pressure in your laptop to rise very quickly and BOOM!
Also Check: Kuta Software Infinite Algebra 2 Systems Of Inequalities Answer Key
Cluster Headaches Migraine And Hypnic Headache
Studies testing prophylactic use of lithium in cluster headaches , migraine attacks and hypnic headache indicate good efficacy.
- Sexual dysfunction
Lithium is known to be responsible for 1â2 kg of weight gain. Weight gain may be a source of low self-esteem for the clinically depressed.
In addition to tremors, lithium treatment appears to be a risk factor for development of parkinsonism symptoms, although the causal mechanism remains unknown.
Most side effects of lithium are dose-dependent. The lowest effective dose is used to limit the risk of side effects.
Voltage And State Of Charge
The voltage of a single LiPo cell depends on its chemistry and varies from about 4.2 V to about 2.73.0 V , where the nominal voltage is 3.6 or 3.7 volts for cells based on lithium-metal-oxides . This compares to 3.63.8 V to 1.82.0 V for those based on lithium-iron-phosphate .
The exact voltage ratings should be specified in product data sheets, with the understanding that the cells should be protected by an electronic circuit that won’t allow them to overcharge nor over-discharge under use.
LiPo battery packs, with cells connected in series and parallel, have separate pin-outs for every cell. A specialized charger may monitor the charge on a per-cell basis so that all cells are brought to the same state of charge .
Read Also: The Segment Addition Postulate Answer Key With Work
How Does A Lithium
3.7 V lithium-ion battery
3.7 V lithium-ion battery
How does this align with my curriculum?
| Topic |
|---|
Learn about the electrochemistry in the batteries that power many of the devices you use every day.
Picture a world without lithium-ion batteries . Need help? Mobile devices wouldnt look the way they do now. Picture huge, heavy cell phones and laptops. Also picture that both of these things are so expensive that only very rich people can afford them. What you are picturing is the 1980s. Scary, isn’t it?
Did you know?
Lithium-ion batteries were first manufactured and produced by SONY in 1991.
Lithium-ion batteries have become a huge part of our mobile culture. They provide power to much of the technology that our society uses.
What Is Reaction Condition Of Li Reacts With O2
Temperature: > 200
Explanation: The ideal environmental conditions for a reaction, such as temperature, pressure, catalysts, and solvent. Catalysts are substances that speed up the pace of a chemical reaction without being consumed or becoming part of the end product. Catalysts have no effect on equilibrium situations.
Don’t Miss: Holt Geometry Chapter 7 Test Form B Answers
Naturally Occurring Nuclides That Are Not Primordial
Some unstable isotopes which occur naturally are not primordial, as they must be constantly regenerated. This occurs by , or by such processes as geonuclear transmutation . Other examples of common naturally occurring but non-primordial nuclides are isotopes of , , and , which are all daughters of uranium decay and are found in uranium ores. The stable isotope 40Ar is actually more common as a radiogenic nuclide than as a primordial nuclide, forming almost 1% of the earth’s , which is regenerated by the of the extremely long-lived radioactive primordial isotope , whose half-life is on the order of a billion years and thus has been generating argon since early in the Earth’s existence.
A similar radiogenic series is derived from the long-lived radioactive primordial nuclide . These nuclides are described as geogenic, meaning that they are decay or fission products of uranium or other actinides in subsurface rocks. All such nuclides have shorter half-lives than their parent radioactive primordial nuclides. Some other geogenic nuclides do not occur in the of 232Th, 235U, or 238U but can still fleetingly occur naturally as products of the of one of these three long-lived nuclides, such as , which makes up about 1014 of all natural .
What Is Reaction Condition Of H2o Reacts With Li

Explanation: The ideal environmental conditions for a reaction, such as temperature, pressure, catalysts, and solvent. Catalysts are substances that speed up the pace of a chemical reaction without being consumed or becoming part of the end product. Catalysts have no effect on equilibrium situations.
Don’t Miss: How Did Geography Spur Industrialization In The Northeast
Periodic Trends Of Lithium
Being on the upper left side of the Periodic Table, lithium has a fairly low electronegativity and electron affinity as compared to the rest of the elements. Also, lithium has high metallic character and subsequently lower nonmetallic character when compared with the other elements. Lithium has a higher atomic radius than most of the elements on the Periodic Table. In compounds lithium has a +1 charge. In its pure form it is soft and silvery white and has a relatively low melting point .
What Is The Chemistry Involved In Lithium
Reduction takes place at the cathode. There, cobalt oxide combines with lithium ions to form lithium-cobalt oxide . The half-reaction is:
CoO2 + Li+ + e- LiCoO2
Oxidation takes place at the anode. There, the graphite intercalation compound LiC6 forms graphite and lithium ions. The half-reaction is:
LiC6 C6 + Li+ + e-
Here is the full reaction :
LiC6 + CoO2 C6 + LiCoO2
Read Also: Core Connections Algebra 2 Chapter 1 Answers
Design Origin And Terminology
Lithium polymer cells have evolved from lithium-ion and lithium-metal batteries. The primary difference is that instead of using a liquid lithium-salt electrolyte held in an organic solvent , the battery uses a solid polymer electrolyte such as poly , poly , poly or poly .
The solid electrolyte can typically be classified as one of three types: dry SPE, gelled SPE and porous SPE. The dry SPE was the first used in prototype batteries, around 1978 by Michel Armand, and 1985 by ANVAR and Elf Aquitaine of France, and Hydro-Québec of Canada. From 1990 several organisations like Mead and Valence in the United States and GS Yuasa in Japan developed batteries using gelled SPEs. In 1996, Bellcore in the United States announced a rechargeable lithium polymer cell using porous SPE.
A typical cell has four main components: positive electrode, negative electrode, separator and electrolyte. The separator itself may be a polymer, such as a microporous film of polyethylene or polypropylene thus, even when the cell has a liquid electrolyte, it will still contain a “polymer” component. In addition to this, the positive electrode can be further divided into three parts: the lithium-transition-metal-oxide , a conductive additive, and a polymer binder of poly . The negative electrode material may have the same three parts, only with carbon replacing the lithium-metal-oxide.
Applying Pressure On Lipo Cells
Unlike lithium-ion cylindrical and prismatic cells, which have a rigid metal case, LiPo cells have a flexible, foil-type case, so they are relatively unconstrained.Moderate pressure on the stack of layers that compose the cell results in increased capacity retention, because the contact between the components is maximised and delamination and deformation is prevented, which is associated with increase of cell impedance and degradation.
LiPo cells provide manufacturers with compelling advantages. They can easily produce batteries of almost any desired shape. For example, the space and weight requirements of mobile devices and notebook computers can be met. They also have a low self-discharge rate, which is about 5% per month.
Recommended Reading: Beth Thomas
Salts And Product Names
Many different lithium salts can be used as medication, including lithium carbonate, lithium acetate, lithium sulfate, lithium citrate, lithium orotate, and lithium gluconate.
Lithium carbonate , sold under several trade names, is the most commonly prescribed, while lithium citrate is also used in conventional pharmacological treatments. Lithium orotate , has been presented as an alternative.Lithium bromide and lithium chloride have been used in the past as table salt however, they fell out of use in the 1940s, when it was discovered they were toxic in those large doses. Many other lithium salts and compounds exist, such as lithium fluoride and lithium iodide, but they are presumed to be as toxic or more so than the chloride and have never been evaluated for pharmacological effects.
What Are The Parts Of A Lithium
A battery is made up of several individual cells that are connected to one another. Each cell contains three main parts: a positive electrode , a negative electrode and a liquid electrolyte.
Just like alkaline dry cell batteries, such as the ones used in clocks and TV remote controls, lithium-ion batteries provide power through the movement of ions. Lithium is extremely reactive in its elemental form. Thats why lithium-ion batteries dont use elemental lithium. Instead, lithium-ion batteries typically contain a lithium-metal oxide, such as lithium-cobalt oxide . This supplies the lithium-ions. Lithium-metal oxides are used in the cathode and lithium-carbon compounds are used in the anode. These materials are used because they allow for intercalation. Intercalation means that the molecules are able to insert something into them. In this case, the electrodes are able to have lithium-ions move easily in and out of their structures.
Read Also: Span Meaning Linear Algebra
Lithium Cells Are Named After The Chemical Composition Of Their Cathode Material
Cells are constructed of several elements, including the cathode, anode, electrolyte, and membrane. The biggest impact on the specs of todays commercially available batteries is made by the chemistry of their cathode materials. That is why battery cells are named after the chemical composition of the materials used in the cathode of a lithium cell.
There are multiple cathode materials to choose from within the Li-ion technology space. The best-known active component of the cathode is cobalt, widely used in batteries for electronics and EVs. Today, battery manufacturers using cobalt are facing serious supply-chain sustainability issues . Cobalt is frequently substituted out with iron , nickel, manganese, and aluminum.
OneCharge batteries are based on LFP cells, the optimal choice for material-handling applications.
Your User Agent Does Not Support The Html5 Audio Element

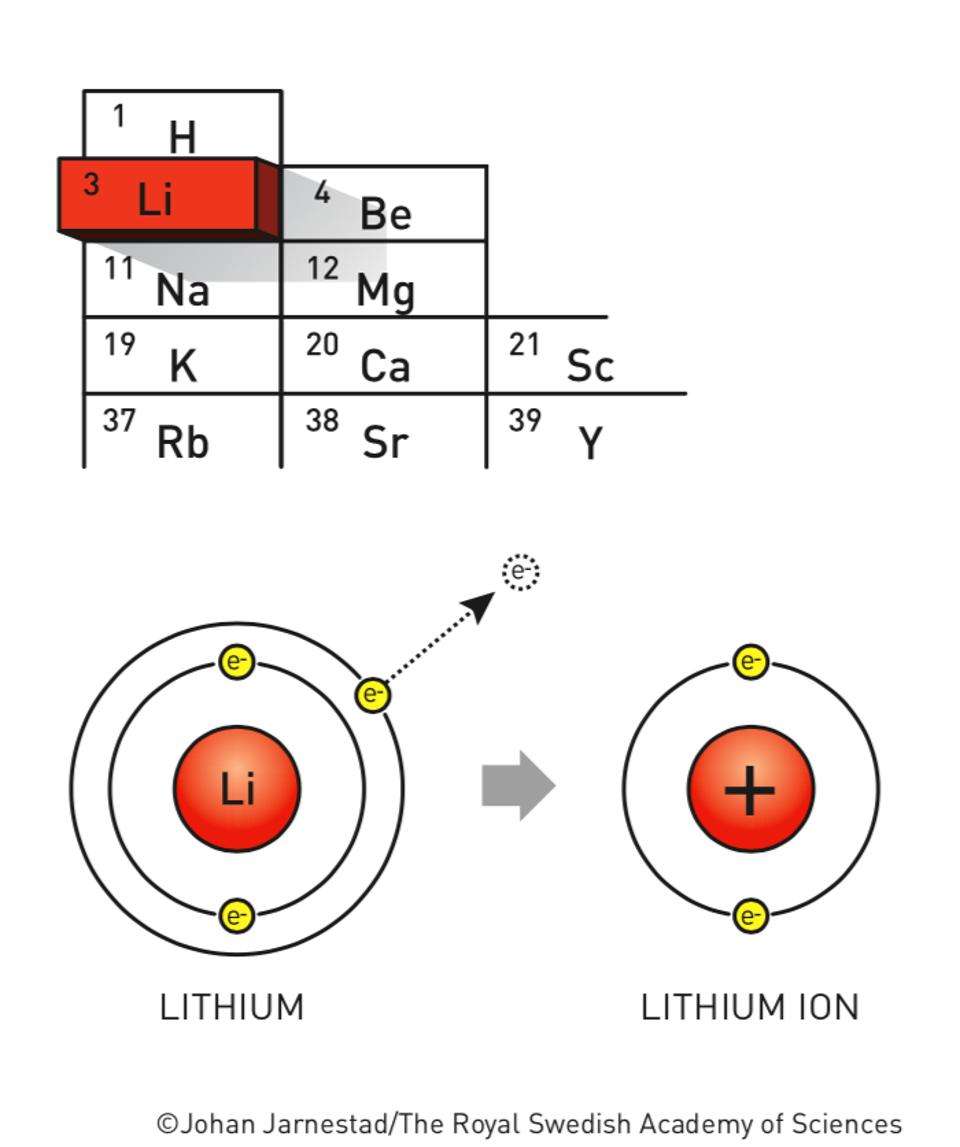
Lithium is a Group 1 element containing just a single valence electron . Group 1 elements are called “alkali metals”. Lithium is a solid only about half as dense as water and lithium metal is the least dense metal. A freshly cut chunk of lithium is silvery, but tarnishes in a minute or so in air to give a grey surface. Its chemistry is dominated by its tendency to lose an electron to form Li+. It is the first element within the second period.
Lithium is mixed with aluminium and magnesium for light-weight alloys, and is also used in batteries, some greases, some glasses, and in medicine.
Lithium does not occur as the free metal in nature because of its high reactivity. Deposits are known all aroun the world. It is a minor component of nearly all igneous rocks and is a component of many natural brines.
You May Like: Michael Jackson’s Biological Children